Microwave-Assisted Sequential One-Pot Synthesis of 8-Substituted Pyrazolo[1,5-a][1,3,5]triazines
Abstract
:1. Introduction
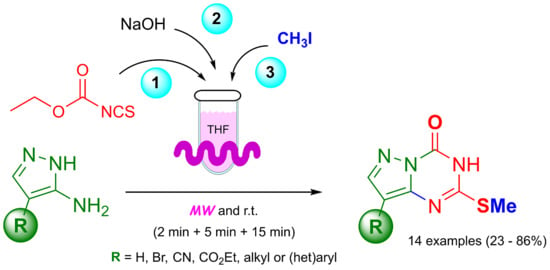
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Chemistry
3.2.1. Synthesis of 2-Thioxo-1H-pyrazolo[1,5-a][1,3,5]triazin-4-one (3a)
3.2.2. General Procedure for the Synthesis of 2-(Methylsulfanyl)pyrazolo[1,5-a][1,3,5]triazin-4(3H)-ones (4a–n)
3.2.3. Synthesis of Pyrazolo[1,5-a][1,3,5]triazines 5 and 6
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Dolzhenko, A.V.; Chu, W.-K. 1,2,4-Triazolo[1,5-a][1,3,5]triazines (5-azapurines): Synthesis and biological activity. Heterocycles 2006, 68, 1723–1759. [Google Scholar] [CrossRef]
- Dolzhenko, A.V.; Chu, W.-K. Pyrazolo[1,5-a][1,3,5]triazines(5-Aza-9-deazapurines): Synthesis and biological activity. Heterocycles 2008, 75, 1575–1622. [Google Scholar] [CrossRef] [Green Version]
- Senga, K.; O’Brien, D.E.; Scholten, M.B.; Novinson, T.; Miller, J.P.; Robins, R.K. Synthesis and enzymic activity of various substituted pyrazolo[1,5-a]-1,3,5-triazines as adenosine cyclic 3′,5′-phosphate phosphodiesterase inhibitors. J. Med. Chem. 1982, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Fujii, S.; Kawamura, H.; Kiyokawa, H.; Yamada, S. Pyrazolotriazines Compounds. U.S. Patent 4,824,834, 25 April 1989. [Google Scholar]
- Lübbers, T.; Angehrn, P.; Gmünder, H.; Herzig, S.; Kulhanek, J. Design, synthesis, and structure–activity relationship studies of ATP analogues as DNA gyrase inhibitors. Bioorg. Med. Chem. Lett. 2000, 10, 821–826. [Google Scholar] [CrossRef]
- Raboisson, P.; Baurand, A.; Cazenave, J.-P.; Gachet, C.; Schultz, D.; Spiess, B.; Bourguignon, J.-J. A general approach toward the synthesis of C-nucleoside Pyrazolo[1,5-a]-1,3,5-triazines and their 3′,5′-bisphosphate C-nucleotide analogues as the first reported in vivo stable P2Y1-receptor antagonists. J. Org. Chem. 2002, 67, 8063–8071. [Google Scholar] [CrossRef]
- Bernard, P.; Raboisson, P.; Joseph, B. Novel Substituted Pyrazolo[1,5-a]-1,3,5-Triazine Derivatives and Their Analogs, Pharmaceutical Compositions Containing Same, Use Thereof as Medicine and Methods for Preparing Same. WO 2004/011464, 5 February 2004. [Google Scholar]
- Guzi, T.J.; Paruch, K. Pyrazolotrazines as Kinase Inhibitors. U.S. Patent 0,187,219, 23 February 2005. [Google Scholar]
- Insuasty, H.; Estrada, M.; Cortés, E.; Quiroga, J.; Insuasty, B.; Abonía, R.; Nogueras, M.; Cobo, J. Regioselective synthesis of novel 4-aryl-2-ethylthio-7-methyl pyrazolo[1,5-a]-[1,3,5]-triazines. Tetrahedron Lett. 2006, 47, 5441–5443. [Google Scholar] [CrossRef]
- Nie, Z.; Perretta, C.; Erickson, P.; Margosiak, S.; Almassy, R.; Lu, J.; Averill, A.; Yager, K.M.; Chu, S. Structure-based design, synthesis, and study of pyrazolo[1,5-a][1,3,5]triazine derivatives as potent inhibitors of protein kinase CK2. Bioorg. Med. Chem. Lett. 2007, 17, 4191–4195. [Google Scholar] [CrossRef]
- Raboisson, P.; Schultz, D.; Muller, C.; Reimund, J.-M.; Pinna, G.; Mathieu, R.; Bernard, P.; Do, Q.-T.; DesJarlais, R.L.; Justiano, H.; et al. Cyclic nucleotide phosphodiesterase type 4 inhibitors: Evaluation of pyrazolo[1,5-a]-1,3,5-triazine ring system as an adenine bioisostere. Eur. J. Med. Chem. 2008, 43, 816–829. [Google Scholar] [CrossRef]
- Popowycz, F.; Fournet, G.; Schneider, C.; Bettayeb, K.; Ferandin, Y.; Lamigeon, C.; Tirado, O.M.; Mateo-Lozano, S.; Notario, V.; Colas, P.; et al. Pyrazolo [1, 5-a]-1, 3, 5-triazine as a purine bioisostere: Access to potent cyclin-dependent kinase inhibitor (R)-roscovitine analogue. J. Med. Chem. 2009, 52, 655–663. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Obitsu, T.; Minamoto, C.; Sugiura, T.; Matsumura, N.; Ueno, S.; Kishi, A.; Katsumata, S.; Nakai, H.; Toda, M. Pyrazolo[1,5-a]pyrimidines, triazolo[1,5-a]pyrimidines and their tricyclic derivatives as corticotropin-releasing factor 1 (CRF1) receptor antagonists. Bioorg. Med. Chem. 2011, 19, 5955–5966. [Google Scholar] [CrossRef]
- Sun, L.; Bera, H.; Chui, W.K. Synthesis of pyrazolo[1,5-a][1,3,5]triazine derivatives as inhibitors of thymidine phosphorylase. Eur. J. Med. Chem. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Bera, H.; Dolzhenko, A.V.; Chiu, G.N.C.; Chui, W.K. Fragment-based approach to the design of 5-chlorouracil-linked-pyrazolo[1,5-a][1,3,5]triazines as thymidine phosphorylase inhibitors. Eur. J. Med. Chem. 2013, 70, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Bera, H.; Lee, M.H.; Sun, L.; Dolzhenko, A.V.; Chui, W.K. Synthesis, anti-thymidine phosphorylase activity and molecular docking of 5-thioxo-[1,2,4]triazolo[1,5-a][1,3,5]triazin-7-ones. Bioorg. Chem. 2013, 50, 34–40. [Google Scholar] [CrossRef]
- Horsley, H.T.; Huang, Q.; Neuss, J.C.; Reuberson, J.T.; Vanderhoydonck, B. Fused Bicyclic Heteroaromatic Derivatives as Kinase Inhibitors and Their Preparation. WO 2015/193169, 12 December 2015. [Google Scholar]
- Webber, S.E.; Tao, X.; Brin, E. CK2 Inhibitors, Compositions and Methods Thereof. WO 2017/223432, 28 December 2017. [Google Scholar]
- Khulood, H.O.; Mazin, A.A.N.; Nermin, S.; Rabah, A.T.S.; Khaled, A.M.A. Design, synthesis and molecular docking of novel pyrazolo[1,5-a][1,3,5]triazine derivatives as CDK2 inhibitors. Bioorg. Chem. 2019, 92, 103239. [Google Scholar] [CrossRef]
- Bongard, J.; Schmitz, A.L.; Wolf, A.; Zischinsky, G.; Pieren, M.; Schellhorn, B.; Bravo-Rodriguez, K.; Schillinger, J.; Koch, U.; Nussbaumer, P.; et al. Chemical validation of DegS as a target for the development of antibiotics with a novel mode of action. ChemMedChem 2019, 14, 1074–1078. [Google Scholar] [CrossRef]
- Kobe, J.; O’rien, D.E.; Robins, R.K.; Novinson, T. The chemistry of 4-hydrazino-7-phenylpyrazolo[1,5-a]-1,3,5-triazines. J. Heterocycl. Chem. 1974, 11, 991–996. [Google Scholar] [CrossRef]
- Kobe, J.; Springer, R.H.; O’Brien, D.E. Pyrazolo [1,5-a] 1,3,5-Triazines. U.S. Patent 3,846,423, 5 November 1974. [Google Scholar]
- Kobe, J.; O’Brien, D.E.; Robins, R.K. 2-Aryl-7-Substituted Pyrazolo [1,5-a] 1,3,5-Triazines. U.S. Patent 3,865,824, 11 February 1975. [Google Scholar]
- Vogel, A.; Troxler, F. Neue Synthesen von Pyrazolo[1,5-a]-s-triazinen. Helv. Chim. Acta 1975, 58, 761–771. [Google Scholar] [CrossRef]
- Senga, K.; Kobe, J.; Robins, R.K.; O’Brien, D.E. Synthesis of 1,3-dialkylpyrazolo [1,5-a]-1,3,5-triazine-2,4-diones isomers of 1,3-dialkylxanthines. J. Heterocycl. Chem. 1975, 12, 893–898. [Google Scholar] [CrossRef]
- Tam, S.Y.K.; Klein, R.S.; Wempen, I.; Fox, J.J. Nucleosides. 112. Synthesis of some new pyrazolo[1,5-a]-1,3,5-triazines and their C-nucleosides. J. Org. Chem. 1979, 44, 4547–4553. [Google Scholar] [CrossRef]
- Chu, C.K.; Watanabe, K.A.; Fox, J.J. Nucleosides. 117. Synthesis of 4-oxo-8-(β-D-ribofuranosyl)-3H-pyrazolo[1,5-a]-1,3,5-triazine (OPTR) via 3-amino-2N-carbamoyl-4-(β-D-ribofuranosyl)pyrazole (ACPR) derivatives. J. Heterocycl. Chem. 1980, 17, 1435–1439. [Google Scholar] [CrossRef]
- Chu, C.K. A facile synthesis of 8-(β-D-ribofuranosyl)-4-thioxo-3H-pyrazolo[1,5-a]-1,3,5-triazine and its α-anomer. J. Heterocycl. Chem. 1984, 21, 389–392. [Google Scholar] [CrossRef]
- Robins, R.K.; Revankar, G.R.; O’Brien, D.E.; Springer, R.H.; Albert, T.N.A.; Senga, K.; Miller, J.P.; Streeter, D.G. Purine analog inhibitors of xanthine oxidase—Structure activity relationships and proposed binding of the molybdenum cofactor. J. Heterocycl. Chem. 1985, 22, 601–634. [Google Scholar] [CrossRef]
- Raboisson, P.; Schultz, D.; Lugnier, C.; Bourguignon, J.-J. Efficient synthesis of 8-substituted pyrazolo[1,5-a]-1,3,5-triazines by regioselective acylation. Tetrahedron Lett. 2002, 43, 9501–9503. [Google Scholar] [CrossRef]
- Raboisson, P.; Schultz, D.; Lugnier, C.; Mathieu, R.; Bourguignon, J.-J. A general approach toward the synthesis of 8-acylamidopyrazolo[1,5-a]-1,3,5-triazines. Tetrahedron Lett. 2003, 44, 703–705. [Google Scholar] [CrossRef]
- Popowycz, F.; Bernard, P.; Raboisson, P.; Joseph, B. Synthesis of 8-substituted pyrazolo[1,5-a]-1,3,5-triazine derivatives by palladium-catalyzed cross-coupling reactions. Synthesis 2007, 367–374. [Google Scholar] [CrossRef]
- Bondock, S.; Tarhoni, A.G.; Fadda, A. heterocyclic synthesis with 4-benzoyl-1-cyanoacetylthiosemicarbazide: Selective synthesis of some thiazole, triazole, thiadiazine, pyrrylthiazole, and pyrazolo[1,5-a]triazine derivatives. Monatsh. Chem. 2008, 139, 153–159. [Google Scholar] [CrossRef]
- El-Mekabaty, A.; Habib, O.M.O.; Moawad, E.B.; Hasel, A.M. Reactivity of 2-thiazolylhydrazonomalononitrile toward carbon and nitrogen nucleophilic reagents: Applications to the synthesis of new heterocycles. J. Heterocycl. Chem. 2016, 53, 1214–1221. [Google Scholar] [CrossRef]
- Lim, F.P.L.; Halcovitch, N.R.; Tiekink, E.R.T.; Dolzhenko, A.V. A new microwave-assisted, three-component reaction of 5-aminopyrazole-4-carboxylates: Selective synthesis of substituted 5-aza-9-deaza-adenines. Tetrahedron 2018, 74, 1868–1879. [Google Scholar] [CrossRef]
- Aggarwal, R.; Kumar, S. 5-Aminopyrazole as precursor in design and synthesis of fused pyrazoloazines. Beilstein J. Org. Chem. 2018, 14, 203–242. [Google Scholar] [CrossRef] [Green Version]
- Lim, F.P.L.; Luna, G.; Tan, K.C.; Tiekink, E.R.T.; Dolzhenko, A.V. A synthesis of new 7-amino-substituted 4-aminopyrazolo[1,5-a][1,3,5]triazines via a selective three-component triazine ring annulation. Tetrahedron 2019, 75, 2322–2329. [Google Scholar] [CrossRef]
- Hédou, D.; Deau, E.; Dubouilh-Benard, C.; Sanselme, M.; Martinet, A.; Chosson, E.; Levacher, V.; Besson, T. Microwave-assisted (3+2) cycloaddition and Suzuki-Miyaura cross-coupling for a concise access to novel polyaromatic scaffolds. Eur. J. Org. Chem. 2013, 7533–7545. [Google Scholar] [CrossRef]
- Deau, E.; Hédou, D.; Chosson, E.; Levacher, V.; Besson, T. Convenient one-pot synthesis of N3-substituted pyrido[2,3-d]-, pyrido[3,4-d]-, pyrido[4,3-d]-pyrimidin-4(3H)-ones, and quinazolin-4(3H)-ones analogs. Tetrahedron Lett. 2013, 54, 3518–3521. [Google Scholar] [CrossRef]
- Harari, M.; Couly, F.; Fruit, C.; Besson, T. Pd-catalyzed and copper assisted regioselective sequential C2 and C7 arylation of thiazolo[5,4-f]quinazolin-9(8H)-one with aryl halides. Org. Lett. 2016, 18, 3282–3285. [Google Scholar] [CrossRef] [PubMed]
- Foucourt, A.; Dubouilh-Benard, C.; Chosson, E.; Corbière, C.; Buquet, C.; Iannelli, M.; Leblond, B.; Marsais, F.; Besson, T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 2010, 66, 4495–4502. [Google Scholar] [CrossRef]
- Lefoix, M.; Mathis, G.; Kleinmann, T.; Truffert, J.-C.; Asseline, U. Pyrazolo[1,5-a]-1,3,5-triazine C-nucleoside as deoxyadenosine analogue: Synthesis, pairing, and resistance to hydrolysis. J. Org. Chem. 2014, 79, 3221–3227. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elie, J.; Fruit, C.; Besson, T. Microwave-Assisted Sequential One-Pot Synthesis of 8-Substituted Pyrazolo[1,5-a][1,3,5]triazines. Molecules 2021, 26, 3540. https://doi.org/10.3390/molecules26123540
Elie J, Fruit C, Besson T. Microwave-Assisted Sequential One-Pot Synthesis of 8-Substituted Pyrazolo[1,5-a][1,3,5]triazines. Molecules. 2021; 26(12):3540. https://doi.org/10.3390/molecules26123540
Chicago/Turabian StyleElie, Jonathan, Corinne Fruit, and Thierry Besson. 2021. "Microwave-Assisted Sequential One-Pot Synthesis of 8-Substituted Pyrazolo[1,5-a][1,3,5]triazines" Molecules 26, no. 12: 3540. https://doi.org/10.3390/molecules26123540
APA StyleElie, J., Fruit, C., & Besson, T. (2021). Microwave-Assisted Sequential One-Pot Synthesis of 8-Substituted Pyrazolo[1,5-a][1,3,5]triazines. Molecules, 26(12), 3540. https://doi.org/10.3390/molecules26123540