Modulatory Effects of Caffeine and Pentoxifylline on Aromatic Antibiotics: A Role for Hetero-Complex Formation
Abstract
:1. Introduction
2. Results
2.1. Antibiotic-Xanthine Interactions: Spectrophotometric and Statistical Modelling Analysis
2.2. Thermal Effects of Antibiotic-Xanthine Interactions
2.3. Antibacterial Activity of Caffeine and Pentoxifylline
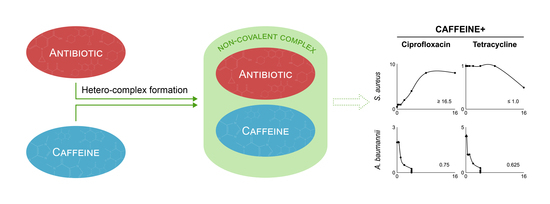
2.4. Modulation of Antibiotic Activity by Caffeine and Pentoxifylline
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. UV-Vis Spectroscopy Measurements
4.3. Quantitative Analysis of Antibiotic-Xanthine Interactions
4.4. Calculations with Statistical-Thermodynamical Model
4.5. Isothermal Titration Calorimetry (ITC)
4.6. Antibacterial Assays
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Cassini, A.; Högberg, L.D.; Plachouras, D.; Quattrocchi, A.; Hoxha, A.; Simonsen, G.S.; Colomb-Cotinat, M.; Kretzschmar, M.E.; Devleesschauwer, B.; Cecchini, M.; et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019, 19, 56–66. [Google Scholar] [CrossRef] [Green Version]
- U.S. Department of Health and Human Services, Centres for Disease Control and Prevention. Antibiotic Resistance Threats in the United States. 2019. Available online: https://www.cdc.gov/drugresistance/pdf/threats-report/2019-ar-threats-report-508.pdf (accessed on 29 April 2021).
- Chen, T.; Wu, D.; Chen, H.; Yan, W.; Yang, D.; Chen, G.; Ma, K.; Xu, D.; Yu, H.; Wang, H.; et al. Clinical characteristics of 113 deceased patients with coronavirus disease 2019: Retrospective study. BMJ 2020, 368, m1295. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Lescure, F.X.; Bouadma, L.; Nguyen, D.; Parisey, M.; Wicky, P.H.; Behillil, S.; Gaymard, A.; Bouscambert-Duchamp, M.; Donati, F.; Le Hingrat, Q.; et al. Clinical and virological data of the first cases of COVID-19 in Europe: A case series. Lancet Infect. Dis. 2020, 20, 697–706. [Google Scholar] [CrossRef] [Green Version]
- MacIntyre, C.R.; Chughtai, A.A.; Barnes, M.; Ridda, I.; Seale, H.; Toms, R.; Heywood, A. The role of pneumonia and secondary bacterial infection in fatal and serious outcomes of pandemic influenza a(H1N1)pdm09. BMC Infect. Dis. 2018, 18, 637. [Google Scholar] [CrossRef] [Green Version]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C. Opinion—Anti-infectives: Where will new antibiotics come from? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef]
- Hutchings, M.; Truman, A.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Gottwalt, S.; Beyer, P.; Butler, M.; Czaplewski, L.; Lienhardt, C.; Moja, L.; Paul, M.; Paulin, S.; Rex, J.H.; et al. Analysis of the clinical antibacterial and antituberculosis pipeline. Lancet Infect. Dis. 2019, 19, e40–e50. [Google Scholar] [CrossRef]
- Meyerhoff, A. U.S. Food and Drug Administration Approval of AmBisome (Liposomal Amphotericin B) for Treatment of Visceral Leishmaniasis. Clin. Infect. Dis. 1999, 28, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Tan, K.R.; Magill, A.J.; Parise, M.E.; Arguin, P.M. Doxycycline for malaria chemoprophylaxis and treatment: Report from the CDC expert meeting on malaria chemoprophylaxis. Am. J. Trop. Med. Hyg. 2011, 84, 517–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imperi, F.; Massai, F.; Ramachandran Pillai, C.; Longo, F.; Zennaro, E.; Rampioni, G.; Visca, P.; Leoni, L. New life for an old Drug: The anthelmintic drug niclosamide inhibits pseudomonas aeruginosa quorum sensing. Antimicrob. Agents Chemother. 2013, 57, 996–1005. [Google Scholar] [CrossRef] [Green Version]
- Bush, K.; Bradford, P.A. β-lactams and β-lactamase inhibitors: An overview. Cold Spring Harb. Perspect. Med. 2016, 6, a025247. [Google Scholar] [CrossRef] [PubMed]
- Stokes, J.M.; MacNair, C.R.; Ilyas, B.; French, S.; Côté, J.P.; Bouwman, C.; Farha, M.A.; Sieron, A.O.; Whitfield, C.; Coombes, B.K.; et al. Pentamidine sensitizes Gram-negative pathogens to antibiotics and overcomes acquired colistin resistance. Nat. Microbiol. 2017, 2, 17028. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, D.C.; Hockenberry, J.; Teplansky, R.; Hartman, T.J. Assessing dietary exposure to caffeine from beverages in the U.S. population using brand-specific versus category-specific caffeine values. Food Chem. Toxicol. 2015, 80, 247–252. [Google Scholar] [CrossRef] [PubMed]
- Reyes, C.M.; Cornelis, M.C. Caffeine in the diet: Country-level consumption and guidelines. Nutrients 2018, 10, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, B.; Roberts, R.S.; Davis, P.; Doyle, L.W.; Barrington, K.J.; Ohlsson, A.; Solimano, A.; Tin, W.; Caffeine for Apnea of Prematurity Trial Group. Caffeine therapy for apnea of prematurity. N. Engl. J. Med. 2006, 354, 2112–2121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derry, C.J.; Derry, S.; Moore, R.A. Caffeine as an analgesic adjuvant for acute pain in adults. Cochrane Database Syst. Rev. 2014, 2017, CD009281. [Google Scholar] [CrossRef] [PubMed]
- Sonka, K.; Susta, M. Diagnosis and management of central hypersomnias. Ther. Adv. Neurol. Disord. 2012, 5, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jull, A.B.; Arroll, B.; Parag, V.; Waters, J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst. Rev. 2012, 12, CD001733. [Google Scholar] [CrossRef]
- McCarty, M.F.; O’Keefe, J.H.; DiNicolantonio, J.J. Pentoxifylline for vascular health: A brief review of the literature. Open Heart 2016, 3, e000365. [Google Scholar] [CrossRef] [Green Version]
- Champion, S.; Lapidus, N.; Cherié, G.; Spagnoli, V.; Oliary, J.; Solal, A.C. Pentoxifylline in heart failure: A meta-analysis of clinical trials. Cardiovasc. Ther. 2014, 32, 159–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gołuński, G.; Woziwodzka, A.; Iermak, I.; Rychłowski, M.; Piosik, J. Modulation of acridine mutagen ICR191 intercalation to DNA by methylxanthines—Analysis with mathematical models. Bioorg. Med. Chem. 2013, 21, 3280–3289. [Google Scholar] [CrossRef]
- Gołuński, G.; Borowik, A.; Derewońko, N.; Kawiak, A.; Rychłowski, M.; Woziwodzka, A.; Piosik, J. Pentoxifylline as a modulator of anticancer drug doxorubicin. Part II: Reduction of doxorubicin DNA binding and alleviation of its biological effects. Biochimie 2016, 123, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Evstigneev, M.P.; Khomich, V.V.; Davies, D.B. Complexation of anthracycline drugs with DNA in the presence of caffeine. Eur. Biophys. J. 2006, 36, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Ulanowska, K.; Piosik, J.; Gwizdek-Wiśniewska, A.; Weģrzyn, G. Formation of stacking complexes between caffeine (1,2,3-trimethylxanthine) and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine may attenuate biological effects of this neurotoxin. Bioorg. Chem. 2005, 33, 402–413. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Gołuński, G.; Wyrzykowski, D.; Kaźmierkiewicz, R.; Piosik, J. Caffeine and other methylxanthines as interceptors of food-borne aromatic mutagens: Inhibition of Trp-P-1 and Trp-P-2 mutagenic activity. Chem. Res. Toxicol. 2013, 26, 1660–1673. [Google Scholar] [CrossRef]
- Woziwodzka, A.; Gwizdek-Wiśniewska, A.; Piosik, J. Caffeine, pentoxifylline and theophylline form stacking complexes with IQ-type heterocyclic aromatic amines. Bioorg. Chem. 2011, 39, 10–17. [Google Scholar] [CrossRef]
- Wikoff, D.; Welsh, B.T.; Henderson, R.; Brorby, G.P.; Britt, J.; Myers, E.; Goldberger, J.; Lieberman, H.R.; O’Brien, C.; Peck, J.; et al. Systematic review of the potential adverse effects of caffeine consumption in healthy adults, pregnant women, adolescents, and children. Food Chem. Toxicol. 2017, 109, 585–648. [Google Scholar] [CrossRef]
- Zdunek, M.; Piosik, J.; Kapuscinski, J. Thermodynamical model of mixed aggregation of ligands with caffeine in aqueous solution. Part II. Biophys. Chem. 2000, 84, 77–85. [Google Scholar] [CrossRef]
- Odds, F.C. Synergy, antagonism, and what the chequerboard puts between them. J. Antimicrob. Chemother. 2003, 52, 1. [Google Scholar] [CrossRef]
- U.S. Department of Health and Human Services and U.S. Department of Agriculture. 2015–2020 Dietary Guidelines for Americans. Available online: https://health.gov/sites/default/files/2019-09/2015-2020_Dietary_Guidelines.pdf (accessed on 29 April 2021).
- Willson, C. The clinical toxicology of caffeine: A review and case study. Toxicol. Rep. 2018, 5, 1140–1152. [Google Scholar] [CrossRef]
- Smith, R.V.; Waller, E.S.; Doluisio, J.T.; Bauza, M.T.; Puri, S.K.; Ho, I.; Lassman, H.B. Pharmacokinetics of orally administered pentoxifylline in humans. J. Pharm. Sci. 1986, 75, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Almeida, A.A.P.; Farah, A.; Silva, D.A.M.; Nunan, E.A.; Glória, M.B.A. Antibacterial activity of coffee extracts and selected coffee chemical compounds against enterobacteria. J. Agric. Food Chem. 2006, 54, 8738–8743. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, S.A.; Salameh, M.M.; Phetsomphou, S.; Yang, H.; Seo, C.W. Application of caffeine, 1,3,7-trimethylxanthine, to control Escherichia coli O157:H7. Food Chem. 2006, 99, 645–650. [Google Scholar] [CrossRef]
- Kang, T.M.; Yuan, J.; Nguyen, A.; Becket, E.; Yang, H.; Miller, J.H. The aminoglycoside antibiotic kanamycin damages DNA bases in Escherichia coli: Caffeine potentiates the DNA-damaging effects of kanamycin while suppressing cell killing by ciprofloxacin in Escherichia coli and Bacillus anthracis. Antimicrob. Agents Chemother. 2012, 56, 3216–3223. [Google Scholar] [CrossRef] [Green Version]
- Yu, P.K.W.; Washington, J.A. Lack of antibacterial activity of pentoxifylline. Antimicrob. Agents Chemother. 1986, 30, 170–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Spini, V.; Dacarro, C.; Gazzani, G. Isolation, identification, and quantification of roasted coffee antibacterial compounds. J. Agric. Food Chem. 2007, 55, 10208–10213. [Google Scholar] [CrossRef]
- Kapuscinski, J.; Ardelt, B.; Piosik, J.; Zdunek, M.; Darzynkiewicz, Z. The modulation of the DNA-damaging effect of polycyclic aromatic agents by xanthines: Part I. Reduction of cytostatic effects of quinacrine mustard by caffeine. Biochem. Pharmacol. 2002, 63, 625–634. [Google Scholar] [CrossRef]
- Gołuński, G.; Borowik, A.; Wyrzykowski, D.; Woziwodzka, A.; Piosik, J. Pentoxifylline as a modulator of anticancer drug doxorubicin. Part I: Reduction of doxorubicin DNA binding. Chem. Biol. Interact. 2015, 242, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Masadeh, M.M.; Alzoubi, K.H.; Khabour, O.F.; Al-Azzam, S.I. Ciprofloxacin-Induced Antibacterial Activity Is Attenuated by Phosphodiesterase Inhibitors. Curr. Ther. Res. Clin. Exp. 2015, 77, 14–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paloncýová, M.; Berka, K.; Otyepka, M. Molecular insight into affinities of drugs and their metabolites to lipid bilayers. J. Phys. Chem. B 2013, 117, 2403–2410. [Google Scholar] [CrossRef] [PubMed]
- Mulani, M.S.; Kamble, E.E.; Kumkar, S.N.; Tawre, M.S.; Pardesi, K.R. Emerging strategies to combat ESKAPE pathogens in the era of antimicrobial resistance: A review. Front. Microbiol. 2019, 10, 539. [Google Scholar] [CrossRef]
- The Pew Charitable Trusts. Antibiotics Currently in Clinical Development. Available online: https://www.pewtrusts.org/-/media/assets/2020/04/antibiotics-currently-in-development-april-2020.xlsx (accessed on 29 April 2021).
- Carrillo, J.A.; Benitez, J. Clinically significant pharmacokinetic interactions between dietary caffeine and medications. Clin. Pharmacokinet. 2000, 39, 127–153. [Google Scholar] [CrossRef]
- Weiser, T.; Weigmann, H. Effect of Caffeine on the Bioavailability and Pharmacokinetics of an Acetylsalicylic Acid-Paracetamol Combination: Results of a Phase I Study. Adv. Ther. 2019, 36, 597–607. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, R.; Fanchamps, A. Effect of caffeine on intestinal absorption of ergotamine in man. Eur. J. Clin. Pharmacol. 1974, 7, 213–216. [Google Scholar] [CrossRef]
- Renner, B.; Clarke, G.; Grattan, T.; Beisel, A.; Mueller, C.; Werner, U.; Kobal, G.; Brune, K. Caffeine accelerates absorption and enhances the analgesic effect of acetaminophen. J. Clin. Pharmacol. 2007, 47, 715–726. [Google Scholar] [CrossRef] [Green Version]
- Alshabi, A.M.; Alkahtani, S.A.; Shaikh, I.A.; Habeeb, M.S. Caffeine modulates pharmacokinetic and pharmacodynamic profiles of pioglitazone in diabetic rats: Impact on therapeutics. Saudi Med. J. 2021, 42, 151–160. [Google Scholar] [CrossRef]
- Fritzsche, H.; Petri, I.; Schütz, H.; Weller, K.; Sedmera, P.; Lang, H. On the interaction of caffeine with nucleic acids. III. 1NMR studies of caffeine-5′-adenosine monophosphate and caffeine-poly(riboadenylate) interactions. Biophys. Chem. 1980, 11, 109–119. [Google Scholar] [CrossRef]
- Clinical and Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 9th ed.; Approved Standard; CLSI document M07-A9; CLSI: Annapolis Junction, MD, USA, 2012. [Google Scholar]
- Berenbaum, M.C. A method for testing for synergy with any number of agents. J. Infect. Dis. 1978, 137, 122–130. [Google Scholar] [CrossRef] [PubMed]






| Sample | CTC, mM | CTA, µM | CC, mM | CCC, mM | CAC, µM | C’A, µM | CA, µM | X’BA, µM | XBA, µM | KAC, M−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 0.00 | 43.18 | 0.00 | 0.000 | 0.00 | 43.18 | 43.18 | 0.00 | 0.00 | - |
| 1 | 0.30 | 43.07 | 0.29 | 0.001 | 1.14 | 41.58 | 41.94 | 1.50 | 1.13 | 60.50 |
| 2 | 0.59 | 42.97 | 0.58 | 0.004 | 2.24 | 40.25 | 40.76 | 2.72 | 2.21 | 56.75 |
| 3 | 1.18 | 42.76 | 1.14 | 0.015 | 4.29 | 38.35 | 38.58 | 4.41 | 4.18 | 48.26 |
| 4 | 1.76 | 42.55 | 1.68 | 0.033 | 6.18 | 38.00 | 36.59 | 4.55 | 5.96 | 33.81 |
| 5 | 2.33 | 42.34 | 2.21 | 0.058 | 7.93 | 35.26 | 34.78 | 7.08 | 7.56 | 42.24 |
| 6 | 2.90 | 42.14 | 2.71 | 0.089 | 9.55 | 34.29 | 33.13 | 7.85 | 9.01 | 38.70 |
| 7 | 4.02 | 41.74 | 3.68 | 0.167 | 12.45 | 31.33 | 30.22 | 10.41 | 11.52 | 40.10 |
| 8 | 5.12 | 41.34 | 4.59 | 0.265 | 14.95 | 28.91 | 27.74 | 12.43 | 13.60 | 40.41 |
| 9 | 7.78 | 40.38 | 6.64 | 0.583 | 19.92 | 24.72 | 22.92 | 15.66 | 17.46 | 38.71 |
| 10 | 10.32 | 39.47 | 8.42 | 0.982 | 23.54 | 19.44 | 19.44 | 20.03 | 20.03 | 45.57 |
| 11 | 15.06 | 37.76 | 11.41 | 1.942 | 28.24 | 12.59 | 14.80 | 25.17 | 22.96 | 55.84 |
| Interaction | KAC (SE), M−1 | ΔH (SE), kJ × mol−1 |
|---|---|---|
| Tetracycline-caffeine | 45.6 (2.5) | −3.17 (0.14) |
| Tetracycline-pentoxifylline | 15.8 (0.6) | −4.00 (0.06) |
| Ciprofloxacin-caffeine | 24.7 (0.9) | −1.44 (0.07) |
| Ciprofloxacin-pentoxifylline | 18.4 (1.0) | −2.01 (0.06) |
| Pathogen | MIC (mg/mL) | |
|---|---|---|
| Caffeine | Pentoxifylline | |
| Gram-positive | ||
| Staphylococcus aureus ATCC 25923 | >16 | >16 |
| Enterococcus faecium ATCC 19433 | >16 | >16 |
| Gram-negative | ||
| Pseudomonas aeruginosa ATCC 27853 | >16 | >16 |
| Escherichia coli ATCC 25922 | 4 | >16 |
| Acinetobacter baumannii ATCC 19606 | 4 | >16 |
| Klebsiella pneumoniae ATCC 700603 | 8 | >16 |
| Enterobacter cloacae ATCC 700323 | 8 | >16 |
| MICA | MICA+caffeine (FICI) | MICA+pentoxifylline (FICI) | |
|---|---|---|---|
| [µg/mL] | |||
| Staphylococcus aureusATCC 25923 | |||
| Ciprofloxacin | 0.5–1 | 8 (≥16.5) | 1 (≥2.25) |
| Tetracycline | 1 | 0.5 (≤1.0) | 1 (≥1.0) |
| Enterococcus faeciumATCC 19433 | |||
| Ciprofloxacin | 2 | 8 (≥4.5) | 2 (≥1.0) |
| Tetracycline | 2 | 0.25 (≤0.625) | 1 (≤1.0) |
| Pseudomonas aeruginosaATCC 27853 | |||
| Ciprofloxacin | 0.5 | 0.25 (≤0.53) | 0.25 (≤1.0) |
| Tetracycline | 64 | 32 (≤0.53) | 128 (≥2.25) |
| Escherichia coliATCC 25922 | |||
| Ciprofloxacin | 0.0156 | 0.078 (1.0) | 0.0156 (≥1.0) |
| Tetracycline | 2 | 1 (1.0) | 2 (≥1.0) |
| Acinetobacter baumanniiATCC 19606 | |||
| Ciprofloxacin | 2 | 0.5 (0.75) | 0.25 (≤0.5) |
| Tetracycline | 4 | 1 (0.625) | 1 (≤0.625) |
| Klebsiella pneumoniaeATCC 700603 | |||
| Ciprofloxacin | 0.5 | 0.125 (0.5) | 0.5 (≥1.0) |
| Tetracycline | 32 | 8 (0.53) | 32 (≥1.0) |
| Enterobacter cloacaeATCC 700323 | |||
| Ciprofloxacin | 0.03125 | 0.03125 (1.03) | 0.0625 (≥2.5) |
| Tetracycline | 4 | 2 (1.0) | 4 (≥1.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woziwodzka, A.; Krychowiak-Maśnicka, M.; Gołuński, G.; Felberg, A.; Borowik, A.; Wyrzykowski, D.; Piosik, J. Modulatory Effects of Caffeine and Pentoxifylline on Aromatic Antibiotics: A Role for Hetero-Complex Formation. Molecules 2021, 26, 3628. https://doi.org/10.3390/molecules26123628
Woziwodzka A, Krychowiak-Maśnicka M, Gołuński G, Felberg A, Borowik A, Wyrzykowski D, Piosik J. Modulatory Effects of Caffeine and Pentoxifylline on Aromatic Antibiotics: A Role for Hetero-Complex Formation. Molecules. 2021; 26(12):3628. https://doi.org/10.3390/molecules26123628
Chicago/Turabian StyleWoziwodzka, Anna, Marta Krychowiak-Maśnicka, Grzegorz Gołuński, Anna Felberg, Agnieszka Borowik, Dariusz Wyrzykowski, and Jacek Piosik. 2021. "Modulatory Effects of Caffeine and Pentoxifylline on Aromatic Antibiotics: A Role for Hetero-Complex Formation" Molecules 26, no. 12: 3628. https://doi.org/10.3390/molecules26123628
APA StyleWoziwodzka, A., Krychowiak-Maśnicka, M., Gołuński, G., Felberg, A., Borowik, A., Wyrzykowski, D., & Piosik, J. (2021). Modulatory Effects of Caffeine and Pentoxifylline on Aromatic Antibiotics: A Role for Hetero-Complex Formation. Molecules, 26(12), 3628. https://doi.org/10.3390/molecules26123628