H2S Removal from Groundwater by Chemical Free Advanced Oxidation Process Using UV-C/VUV Radiation
Abstract
:1. Introduction
2. Results
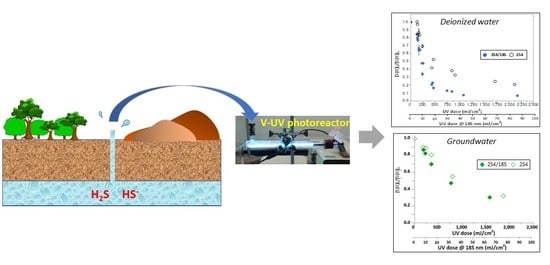
2.1. Photochemistry of Sulfide in Synthetic Solutions
2.2. Photochemistry of Sulfide in Natural Groundwater
2.3. Energy Demand for Photochemistry
3. Materials and Methods
3.1. Chemicals
3.2. Experimental Setup
3.3. Photodegradation Experiments
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- American Water Works Association. Water Quality and Treatment A Handbook of Community Water Supplies, 2nd ed.; Precious, S., Ed.; American Water Works Association: Denver, CO, USA, 1999. [Google Scholar]
- Antonopoulou, M.; Evgenidou, E.; Lambropoulou, D.; Konstantinou, I. A review on advanced oxidation processes for the removal of taste and odor compounds from aqueous media. Water Res. 2014, 53, 215–234. [Google Scholar] [CrossRef] [PubMed]
- Water Environment Research Foundation (WERF). Identifying and Controlling Municipal Wastewater Odor Phase I: Literature Search and Review; IWA Publishing: London, UK, 2003. [Google Scholar]
- CCOHS: Databases. Available online: https://www.ccohs.ca/products/databases/ (accessed on 27 March 2013).
- Von Gunten, U. Ozonation of drinking water: Part II. Disinfection and by-product formation in presence of bromide, iodide or chlorine. Water Res. 2003, 37, 1469–1487. [Google Scholar] [CrossRef]
- Mark, G.; Naumov, S.; von Sonntag, C. The reaction of ozone with bisulfide (HS-/) in aqueous solution-mechanistic aspects. Ozone Sci. Eng. 2011, 33, 37–41. [Google Scholar] [CrossRef]
- Zhang, X.L.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Odor control in lagoons. J. Environ. Manag. 2013, 124, 62–71. [Google Scholar] [CrossRef]
- Duranceau, S.J.; Trupiano, V.M.; Lowenstine, M.; Whidden, S.; Hopp, J. Innovative Hydrogen Sulfide Treatment Methods: Moving Beyond Packed Tower Aeration. Fla. Water Resour. J. 2010, 7, 4–14. [Google Scholar]
- Firer, D.; Friedler, E.; Lahav, O. Control of sulfide in sewer systems by dosage of iron salts: Comparison between theoretical and experimental results, and practical implications. Sci. Total Environ. 2008, 392, 145–156. [Google Scholar] [CrossRef]
- Glaze, W.H.; Kang, J.W.; Chapin, D.H. The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. Eng. 1987, 9, 335–352. [Google Scholar] [CrossRef]
- Thiruvenkatachari, R.; Vigneswaran, S.; Moon, I.S. A review on UV/TiO2 photocatalytic oxidation process. Korean J. Chem. Eng. 2008, 25, 64–72. [Google Scholar] [CrossRef]
- Tzvi, Y.; Paz, Y. Highly efficient method for oxidation of dissolved hydrogen sulfide in water, utilizing a combination of UVC light and dissolved oxygen. J. Photochem. Photobiol. A Chem. 2019, 372, 63–70. [Google Scholar] [CrossRef]
- Gonzalez, M.G.; Oliveros, E.; Wörner, M.; Braun, A.M. Vacuum-ultraviolet photolysis of aqueous reaction systems. J. Photochem. Photobiol. C Photochem. Rev. 2004, 5, 225–246. [Google Scholar] [CrossRef]
- Zoschke, K.; Börnick, H.; Worch, E. Vacuum-UV radiation at 185 nm in water treatment—A review. Water Res. 2014, 52, 131–145. [Google Scholar] [CrossRef]
- Alfiya, Y.; Friedler, E.; Westphal, J.; Olsson, O.; Dubowski, Y. Photodegradation of micropollutants using V-UV/UV-C processes; Triclosan as a model compound. Sci. Total Environ. 2017, 601–602, 397–404. [Google Scholar] [CrossRef]
- Kutschera, K.; Börnick, H.; Worch, E. Photoinitiated oxidation of geosmin and 2-methylisoborneol by irradiation with 254 nm and 185 nm UV light. Water Res. 2009, 43, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Hennings, U.; Pötschke, L.; Wietor, C.; Bringmann, S.; Braun, N.; Hayashi, D.; Linnemann, V.; Pinnekamp, J. Improving vacuum-UV (VUV) photolysis of organic compounds in water with a phosphor converted xenon excimer lamp emitting at 193 nm. Water Sci. Technol. 2016, 74, 888–895. [Google Scholar] [CrossRef]
- Sobol, S.; Tzabar, N.; Grossman, G. Miniature Piezoelectric Compressor for Joule-Thomson Cryocoolers. Phys. Procedia 2015, 67, 423–427. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Bu, Y.; Chen, B.; Sadiq, R. Removal of urea from swimming pool water by UV/VUV: The roles of additives, mechanisms, influencing factors, and reaction products. Water Res. 2019, 161, 89–97. [Google Scholar] [CrossRef]
- Matsushita, T.; Sugita, W.; Ishikawa, T.; Shi, G.; Nishizawa, S.; Matsui, Y.; Shirasaki, N. Prediction of 1,4-dioxane decomposition during VUV treatment by model simulation taking into account effects of coexisting inorganic ions. Water Res. 2019, 164, 114918. [Google Scholar] [CrossRef] [PubMed]
- Dubowski, Y.; Alfiya, Y.; Gilboa, Y.; Sabach, S.; Friedler, E. Removal of organic micropollutants from biologically treated greywater using continuous-flow vacuum-UV/UVC photo-reactor. Environ. Sci. Pollut. Res. 2020, 27, 7578–7587. [Google Scholar] [CrossRef]
- Duca, C.; Imoberdorf, G.; Mohseni, M. Effects of inorganics on the degradation of micropollutants with vacuum UV (VUV) advanced oxidation. J. Environ. Sci. Heal. Part A Toxic Hazard. Subst. Environ. Eng. 2017, 52, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Weeks, J.L.; Meaburn, G.M.A.C.; Gordon, S. Absorption Coefficients of Liquid Water and Aqueous Solutions in the Far Ultraviolet. Radiat. Res. 1963, 19, 559. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.Y.; Gu, D.H.; Tan, J.; Dong, W.B.; Hou, H.Q. Photolysis of low concentration H2S under UV/VUV irradiation emitted from microwave discharge electrodeless lamps. Chemosphere 2008, 71, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Napso, S.; Rein, D.M.; Khalfin, R.; Kleinerman, O.; Cohen, Y. Cellulose gel dispersion: From pure hydrogel suspensions to encapsulated oil-in-water emulsions. Colloids Surf. B Biointerfaces 2016, 137, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Zhang, G.; Pan, H. Experimental study on ozone photolytic and photocatalytic degradation of H 2S using continuous flow mode. J. Hazard. Mater. 2012, 199–200, 255–261. [Google Scholar] [CrossRef]
- Xu, J.; Li, C.; Liu, P.; He, D.; Wang, J.; Zhang, Q. Photolysis of low concentration H2S under UV/VUV irradiation emitted from high frequency discharge electrodeless lamps. Chemosphere 2014, 109, 202–207. [Google Scholar] [CrossRef]
- Finlayson-Pitts, B.J.; Pitts, J.N., Jr. Chemistry of the Upper and Lower Atmosphere; Elsevier: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Mazellier, P.; Busset, C.; Delmont, A.; De Laat, J. A comparison of fenuron degradation by hydroxyl and carbonate radicals in aqueous solution. Water Res. 2007, 41, 4585–4594. [Google Scholar] [CrossRef] [PubMed]
- Mehrvar, M.; Anderson, W.A.; Moo-Young, M. Photocatalytic degradation of aqueous organic solvents in the presence of hydroxyl radical scavengers. Int. J. Photoenergy 2002, 3, 187–191. [Google Scholar] [CrossRef]
- Nickelsen, M.G.; Cooper, W.J.; Secker, D.A.; Rosocha, L.A.; Kurucz, C.N.; Waite, T.D. Kinetic modeling and simulation of PCE and TCE removal in aqueous solutions by electron-beam irradiation. Radiat. Phys. Chem. 2002, 65, 579–587. [Google Scholar] [CrossRef]
- Liao, C.H.; Kang, S.F.; Wu, F.A. Hydroxyl radical scavenging role of chloride and bicarbonate ions in the H2O2/UV process. Chemosphere 2001, 44, 1193–1200. [Google Scholar] [CrossRef]
- Saran, M.; Beck-Speier, I.; Fellerhoff, B.; Bauer, G. Phagocytic killing of microorganisms by radical processes: Consequences of the reaction of hydroxyl radicals with chloride yielding chlorine atoms. Free Radic. Biol. Med. 1999, 26, 482–490. [Google Scholar] [CrossRef]
- Mora, A.S.; Mohseni, M. Temperature dependence of the absorbance of 185 nm photons by water and commonly occurring solutes and its influence on the VUV advanced oxidation process. Environ. Sci. Water Res. Technol. 2018, 4, 1303–1309. [Google Scholar] [CrossRef]
- Parsons, S. Advanced Oxidation Processes for Water and Wastewater Treatment; IWA Publishing: London, UK, 2015; Volume 4. [Google Scholar]
- Gągol, M.; Soltani, R.D.C.; Przyjazny, A.; Boczkaj, G. Effective degradation of sulfide ions and organic sulfides in cavitation-based advanced oxidation processes (AOPs). Ultrason. Sonochem. 2019, 58, 104610. [Google Scholar] [CrossRef] [PubMed]
- Lahav, O.; Sagiv, A.; Friedler, E. A different approach for predicting H2S(g) emission rates in gravity sewers. Water Res. 2006, 40, 259–266. [Google Scholar] [CrossRef] [PubMed]
- APHA. Standard Methods for Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998; ISBN 0875530478. [Google Scholar]
- Crittenden, J.C.; Trussell, R.R.; Hand, D.W.; Howe, K.J.; Tchobanoglous, G. Principles of Reactor Analysis and Mixing. In MWH’s Water Treatment; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 287–390. ISBN 9781118131473. [Google Scholar]
- Friedler, E.; Gilboa, Y. Performance of UV disinfection and the microbial quality of greywater effluent along a reuse system for toilet flushing. Sci. Total Environ. 2010, 408, 2109–2117. [Google Scholar] [CrossRef] [PubMed]
- Rahn, R.O.; Stefan, M.I.; Bolton, J.R.; Goren, E.; Shaw, P.-S.; Lykke, K.R. Quantum Yield of the Iodide–Iodate Chemical Actinometer: Dependence on Wavelength and Concentration. Photochem. Photobiol. 2003, 78, 146. [Google Scholar] [CrossRef]
- Yang, L.; Li, M.; Li, W.; Bolton, J.R.; Qiang, Z. A Green Method to Determine VUV (185 nm) Fluence Rate Based on Hydrogen Peroxide Production in Aqueous Solution. Photochem. Photobiol. 2018, 94, 821–824. [Google Scholar] [CrossRef] [PubMed]






| Parameter | Tsofar | Faran |
|---|---|---|
| pH | 7 | 7.03 |
| Alkalinity (mg/L-CaCO3) | 293 | 259 |
| E.C. (µS/cm) | 3760 | 1410 |
| O.D. 405 (Abs./cm) | 0.012 | 0.002 |
| O.D. 254 (Abs./cm) | 0.171 | 0.031 |
| Turbidity (NTU) | 21.1 | 3.9 |
| H2S mg-S/L | 10.5 | 1.7 |
| SO42− mg-SO4/L | 625.7 | 681.4 |
| Cl mg/L | 429.9 | 378.6 |
| Total hardness (mg-CaCO3/L) | 980 | 1000 |
| Ca2+ mg/L | 0 | 272 |
| Mg2+ mg/L | 238 | 77.8 |
| TOC (mg/L) | 1.168 | 0.6867 |
| DOC (mg/L) | 1.085 | 0.3735 |
| Total N (mg/L) | 1.85 | 0.6356 |
| Concentration a | k (·OH) | ·OH Scavenging | ε185nm | extinction (185 nm) | ||
|---|---|---|---|---|---|---|
| mg/L | M | s−1 M−1 | s−1 | M−1 cm−1 | cm−1 | |
| HCO3− | 259–293 | (4.5–4.8) × 10−3 | 8.50 × 106 [29,30,31] | (3.6–4.1) × 104 | 269 [22] | 1.14–1.29 |
| Cl− | 378.6–429.9 | (1.1–1.2) × 10−2 | 3.00 × 109 [32,33] | (3.2–6.3) × 107 | 3063 [34] | 32.67–37.1 |
| SO42− | 681.4–625.7 | (6.5–7.1) × 10−3 | - | - | 146 [34] | 0.95–1.04 |
| WATER | 55.4 | - | - | 0.029 | 1.60 [34] | |
| DOC | 0.3735–1.085 | (3.1–9.0) × 10−5 | 6.60 × 108 [31] | (2.1–5.9) × 104 | 1402 [34] | 0.04–0.13 |
| Sulfide Dissolved in | Lamp Type | UV Dose Required for 50% Removal at 254 nm Wavelengths (mJ/cm2) | UV Dose Required for 50% Removal at 185 nm Wavelengths (mJ/cm2) | Energy Demand for 50% Removal (kWh/m3) |
|---|---|---|---|---|
| DIW | 254/185 | 238 (±12) | 45 (±1.7) | 1.2 |
| DIW | 254 | 417 (±36) | 3.0 | |
| Faran | 254/185 | 317 (±20) | 70 (±2.8) | 2.4 |
| Faran | 254 | 286 (±12) | 2.4 | |
| Faran-oxygen free | 254/185 | 690 (±14) | 131 (±2.0) | 6.1 |
| Faran-oxygen free | 254 | 1045 (±72) | 11 | |
| Tsofar | 254/185 | 398 (±34) | 77 (±4.8) | 2.7 |
| Tsofar | 254 | 248 (±27) | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gilboa, Y.; Alfiya, Y.; Sabach, S.; Friedler, E.; Dubowski, Y. H2S Removal from Groundwater by Chemical Free Advanced Oxidation Process Using UV-C/VUV Radiation. Molecules 2021, 26, 4016. https://doi.org/10.3390/molecules26134016
Gilboa Y, Alfiya Y, Sabach S, Friedler E, Dubowski Y. H2S Removal from Groundwater by Chemical Free Advanced Oxidation Process Using UV-C/VUV Radiation. Molecules. 2021; 26(13):4016. https://doi.org/10.3390/molecules26134016
Chicago/Turabian StyleGilboa, Yael, Yuval Alfiya, Sara Sabach, Eran Friedler, and Yael Dubowski. 2021. "H2S Removal from Groundwater by Chemical Free Advanced Oxidation Process Using UV-C/VUV Radiation" Molecules 26, no. 13: 4016. https://doi.org/10.3390/molecules26134016
APA StyleGilboa, Y., Alfiya, Y., Sabach, S., Friedler, E., & Dubowski, Y. (2021). H2S Removal from Groundwater by Chemical Free Advanced Oxidation Process Using UV-C/VUV Radiation. Molecules, 26(13), 4016. https://doi.org/10.3390/molecules26134016