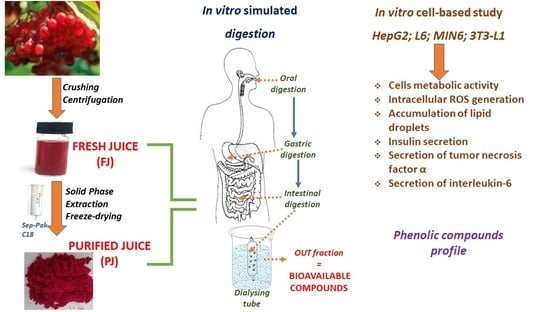
The Effect of Simulated In Vitro Digestion on Biological Activity of Viburnum opulus Fruit Juices
Abstract
:1. Introduction
2. Results
2.1. The Influence of In-Vitro-Digested V. opulus Juice Samples on Metabolic Activity of Cells
2.2. The Effect of Digested V. opulus Juice Samples on Biological Activity of HepG2 Cells
2.3. The Effect of Digested V. opulus Juice Samples on Biological Activity of L6 Cells
2.4. The Effect of Digested V. opulus Juice Samples on Insulin Secretion of MIN6 Cells
2.5. The Effect of Digested V. opulus Juice Samples on Adipogenesis of 3T3-L1 Cells
2.6. Hydroxycinnamic Profiles of V. opulus Fresh (FJ) and Purified (PJ) Juice before and after In Vitro Digestion
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of V. opulus Samples
4.3. Simulated Gastrointestinal Digestion In Vitro
4.4. Identification and Quantification of Phenolic Compounds by UPLC–PDA-Q/TOF-MS
4.5. Cell Culture and Exposure Conditions
4.6. Cell Viability
4.7. Detection of Intracellular Reactive Oxygen Species Generation
4.8. Determination of Lipid Accumulation and Fatty Acid Uptake
4.9. Glucose Uptake
4.10. Insulin Secretion
4.11. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kajszczak, D.; Zakłos-Szyda, M.; Podsędek, A. Viburnum opulus L.—A review of phytochemistry and biological effects. Nutrients 2020, 12, 1–30. [Google Scholar] [CrossRef]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant properties of European cranberrybush fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef]
- Perova, I.B.; Zhogova, A.A.; Cherkashin, A.V.; Éller, K.I.; Ramenskaya, G. V Biologically active substances from European guelder berry fruits. Pharm. Chem. J. 2014, 48, 332–339. [Google Scholar] [CrossRef]
- Baschali, A.; Tsakalidou, E.; Kyriacou, A.; Karavasiloglou, N.; Matalas, A.L. Traditional low-alcoholic and non-alcoholic fermented beverages consumed in European countries: A neglected food group. Nutr. Res. Rev. 2017, 30, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Bojek, U.; Rywaniak, J.; Bernat, P.; Podsędek, A.; Kajszczak, D.; Sadowska, B. An In vitro study of the effect of Viburnum opulus extracts on key processes in the development of Staphylococcal infections. Molecules 2021, 26, 1758. [Google Scholar] [CrossRef]
- Sagdic, O.; Aksoy, A.; Ozkan, G. Evaluation of the antibacterial and antioxidant potentials of cranberry (gilaburu, Viburnum opulus L.) fruit extract. Acta Aliment. 2006, 35, 487–492. [Google Scholar] [CrossRef]
- Česonienė, L.; Daubaras, R.; Kraujalytė, V.; Venskutonis, P.R.; Šarkinas, A. Antimicrobial activity of Viburnum opulus fruit juices and extracts. J. Verbrauch. Leb. 2014, 9, 129–132. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Majewska, I.; Redzynia, M.; Koziołkiewicz, M. Antidiabetic effect of polyphenolic extracts from selected edible plants as α-amylase, α-glucosidase and PTP1B inhibitors, and β pancreatic cells cytoprotective agents-a comparative study. Curr. Top. Med. Chem. 2015, 15, 2431–2444. [Google Scholar] [CrossRef]
- Podsędek, A.; Zakłos-Szyda, M.; Polka, D.; Sosnowska, D. Effects of Viburnum opulus fruit extracts on adipogenesis of 3T3-L1 cells and lipase activity. J. Funct. Foods 2020, 73, 104111. [Google Scholar] [CrossRef]
- Zakłos-Szyda, M.; Pietrzyk, N.; Szustak, M.; Podsędek, A. Viburnum opulus L. juice phenolics inhibit mouse 3T3-L1 cells adipogenesis and pancreatic lipase activity. Nutrients 2020, 12, 1–29. [Google Scholar] [CrossRef]
- Polka, D.; Podsedek, A. Phenolics composition and antioxidant capacity of guelder rose fruit, flower and bark extracts. Biotechnol. Food Sci. 2019, 83, 37–46. [Google Scholar]
- Soylak, A.; Elci, L.; Saracoglu, S.; Divrikli, U. Chemical analysis of fruit juice of European cranberrybush (Viburnum opulus) from Kayseri-Turkey. Asian J. Chem. 2002, 14, 135–138. [Google Scholar]
- Lachowicz, S.; Oszmianski, J. The influence of addition of cranberrybush juice to pear juice on chemical composition and antioxidant properties. J. Food Sci. Technol. 2018, 55, 3399–3407. [Google Scholar] [CrossRef] [Green Version]
- Çemtekİn, B.; Kilinç, E.; Karabacak, L.; Dağtekİn, T. Aa evaluationof guelder rose (Viburnum opulus L.) and hawthorn (Crataegus monogyna) concentrates as alternative antioxidant sources to BHT and nitrite in poultry meat model system. Sci. Pap. Ser. D Anim. Sci. 2019, LXII, 217–227. [Google Scholar]
- Zakłos-Szyda, M.; Pawlik, N.; Polka, D.; Nowak, A.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus fruit phenolic compounds as cytoprotective agents able to decrease free fatty acids and glucose uptake by Caco-2 cells. Antioxidants 2019, 8, 262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakłos-Szyda, M.; Kowalska-Baron, A.; Pietrzyk, N.; Drzazga, A. Evaluation of Viburnum opulus L. fruit phenolics cytoprotective potential on insulinoma MIN6 cells relevant for diabetes mellitus and obesity. Antioxidants 2020, 9, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Pietrzyk, N.; Zakłos-Szyda, M.; Koziołkiewicz, M.; Podsędek, A. Viburnum opulus L. fruit phenolic compounds protect against FFA-induced steatosis of HepG2 cells via AMPK pathway. J. Funct. Foods 2021, 80, 104437. [Google Scholar] [CrossRef]
- Karakurt, S.; Abuşoğlu, G.; Arituluk, Z.C. Comparison of anticarcinogenic properties of Viburnum opulus and its active compound p-coumaric acid on human colorectal carcinoma. Turkish J. Biol. 2020, 44, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Barak, T.H.; Celep, E.; Yesilada, E. Influence of in vitro human digestion on the bioavailability of phenolic content and antioxidant activity of Viburnum opulus L. (European cranberry) fruit extracts. Ind. Crop. Prod. 2019, 131, 62–69. [Google Scholar] [CrossRef]
- Stanisavljević, N.; Samardžić, J.; Janković, T.; Šavikin, K.; Mojsin, M.; Topalović, V.; Stevanović, M. Antioxidant and antiproliferative activity of chokeberry juice phenolics during in vitro simulated digestion in the presence of food matrix. Food Chem. 2015, 175, 516–522. [Google Scholar] [CrossRef]
- Ombra, M.N.; Fratianni, F.; Granese, T.; Cardinale, F.; Cozzolino, A.; Nazzaro, F. In vitro antioxidant, antimicrobial and anti-proliferative activities of purple potato extracts (Solanum tuberosum cv Vitelotte noire) following simulated gastro-intestinal digestion. Nat. Prod. Res. 2015, 29, 1087–1091. [Google Scholar] [CrossRef]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef]
- Mantena, S.K.; King, A.L.; Andringa, K.K.; Eccleston, H.B.; Bailey, S.M. Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 2008, 44, 1259–1272. [Google Scholar] [CrossRef] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased oxidative stress in obesity and its impact on metabolic syndrome. J. Clin. Investig. 2004, 114, 1752–1761. [Google Scholar] [CrossRef]
- Reynoso, R.; Salgado, L.M.; Calderón, V. High levels of palmitic acid lead to insulin resistance due to changes in the level of phosphorylation of the insulin receptor and insulin receptor substrate-1. Mol. Cell. Biochem. 2003, 246, 156–162. [Google Scholar] [CrossRef]
- Cao, J.; Feng, X.X.; Yao, L.; Ning, B.; Yang, Z.X.; Fang, D.L.; Shen, W. Saturated free fatty acid sodium palmitate-induced lipoapoptosis by targeting glycogen synthase kinase-3β activation in human liver cells. Dig. Dis. Sci. 2014, 59, 346–357. [Google Scholar] [CrossRef]
- Tumova, J.; Andel, M.; Trnka, J. Excess of free fatty acids as a cause of metabolic dysfunction in skeletal muscle. Physiol. Res. 2016, 65, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Rachek, L.I. Free fatty acids and skeletal muscle insulin resistance; Prog. Mol. Biol. Transl. Sci. 2014, 121, 267–292. [Google Scholar]
- Sawada, K.; Kawabata, K.; Yamashita, T.; Kawasaki, K.; Yamamoto, N.; Ashida, H. Ameliorative effects of polyunsaturated fatty acids against palmitic acid-induced insulin resistance in L6 skeletal muscle cells. Lipids Health Dis. 2012, 11, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Jiang, J.G. Protective effects of plant-derived flavonoids on hepatic injury. J. Funct. Foods 2018, 44, 283–291. [Google Scholar] [CrossRef]
- Gião, M.S.; Pestana, D.; Faria, A.; Guimarães, J.T.; Pintado, M.E.; Calhau, C.; Azevedo, I.; Malcata, F.X. Effects of extracts of selected medicinal plants upon hepatic oxidative stress. J. Med. Food 2010, 13, 131–136. [Google Scholar] [CrossRef]
- Villalpando-Arteaga, E.V.; Mendieta-Condado, E.; Esquivel-Solís, H.; Canales-Aguirre, A.A.; Gálvez-Gastélum, F.J.; Mateos-Díaz, J.C.; Rodríguez-González, J.A.; Márquez-Aguirre, A.L. Hibiscus sabdariffa L. aqueous extract attenuates hepatic steatosis through down-regulation of PPAR-γ and SREBP-1c in diet-induced obese mice. Food Funct. 2013, 4, 618–626. [Google Scholar] [CrossRef]
- Sousa, J.N.; Paraíso, A.F.; Andrade, J.M.O.; Lelis, D.F.; Santos, E.M.; Lima, J.P.; Monteiro-Junior, R.S.; D’Angelo, M.F.S.V.; de Paula, A.M.B.; Guimarães, A.L.S.; et al. Oral gallic acid improve liver steatosis and metabolism modulating hepatic lipogenic markers in obese mice. Exp. Gerontol. 2020, 134, 110881. [Google Scholar] [CrossRef] [PubMed]
- Żyżelewicz, D.; Zakłos-Szyda, M.; Juśkiewicz, J.; Bojczuk, M.; Oracz, J.; Budryn, G.; Miśkiewicz, K.; Krysiak, W.; Zduńczyk, Z.; Jurgoński, A. Cocoa bean (Theobroma cacao L.) phenolic extracts as PTP1B inhibitors, hepatic HepG2 and pancreatic β-TC3 cell cytoprotective agents and their influence on oxidative stress in rats. Food Res. Int. 2016, 89, 946–957. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, Y.; Mi, S.; Fan, Q.; Sun, X.; Deng, B.; Wu, G.; Li, Y.; Zhou, Q.; Ruan, Z. Hepatoprotective effect of chlorogenic acid against chronic liver injury in inflammatory rats. J. Funct. Foods 2019, 62, 103540. [Google Scholar] [CrossRef]
- Inada, K.O.P.; Silva, T.B.R.; Lobo, L.A.; Domingues, R.M.C.P.; Perrone, D.; Monteiro, M. Bioaccessibility of phenolic compounds of jaboticaba (Plinia jaboticaba) peel and seed after simulated gastrointestinal digestion and gut microbiota fermentation. J. Funct. Foods 2020, 67, 103851. [Google Scholar] [CrossRef]
- Correa-Betanzo, J.; Allen-Vercoe, E.; McDonald, J.; Schroeter, K.; Corredig, M.; Paliyath, G. Stability and biological activity of wild blueberry (Vaccinium angustifolium) polyphenols during simulated in vitro gastrointestinal digestion. Food Chem. 2014, 165, 522–531. [Google Scholar] [CrossRef]
- Mosele, J.I.; Macià, A.; Romero, M.P.; Motilva, M.J.; Rubió, L. Application of in vitro gastrointestinal digestion and colonic fermentation models to pomegranate products (juice, pulp and peel extract) to study the stability and catabolism of phenolic compounds. J. Funct. Foods 2015, 14, 529–540. [Google Scholar] [CrossRef]
- Podsędek, A.; Majewska, I.; Redzynia, M.; Sosnowska, D.; Koziołkiewicz, M. In vitro inhibitory effect on digestive enzymes and antioxidant potential of commonly consumed fruits. J. Agric. Food Chem. 2014, 62, 4610–4617. [Google Scholar] [CrossRef]
- Boaventura, B.C.B.; Amboni, R.D. de M.C.; da Silva, E.L.; Prudencio, E.S.; Di Pietro, P.F.; Malta, L.G.; Polinati, R.M.; Liu, R.H. Effect of in vitro digestion of yerba mate (Ilex paraguariensis A. St. Hil.) extract on the cellular antioxidant activity, antiproliferative activity and cytotoxicity toward HepG2 cells. Food Res. Int. 2015, 77, 257–263. [Google Scholar]
- Jiao, X.; Li, B.; Zhang, Q.; Gao, N.; Zhang, X.; Meng, X. Effect of in vitro-simulated gastrointestinal digestion on the stability and antioxidant activity of blueberry polyphenols and their cellular antioxidant activity towards HepG2 cells. Int. J. Food Sci. Technol. 2018, 53, 61–71. [Google Scholar] [CrossRef]
- Tagliazucchi, D.; Verzelloni, E.; Bertolini, D.; Conte, A. In vitro bio-accessibility and antioxidant activity of grape polyphenols. Food Chem. 2010, 120, 599–606. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, F.; Ju, X.; Li, Z.; Wang, L. Lipid-lowering effects and intestinal transport of polyphenol extract from digested buckwheat in Caco-2/HepG2 coculture models. J. Agric. Food Chem. 2020, 68, 4205–4214. [Google Scholar] [CrossRef]
- Varshney, R.; Mishra, R.; Das, N.; Sircar, D.; Roy, P. A comparative analysis of various flavonoids in the regulation of obesity and diabetes: An in vitro and in vivo study. J. Funct. Foods 2019, 59, 194–205. [Google Scholar] [CrossRef]
- Gumienna, M.; Lasik, M.; Czarnecki, Z. Bioconversion of grape and chokeberry wine polyphenols during simulated gastrointestinal in vitro digestion. Int. J. Food Sci. Nutr. 2011, 62, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Olthof, M.R.; Hollman, P.C.H.; Katan, M.B. Chlorogenic acid and caffeic acid are absorbed in humans. J. Nutr. 2001, 131, 66–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farah, A.; Guigon, F.; Trugo, L.C. 5-Caffeoylquinic acid digestibility in human digestive fluids. In Proceedings of the 21st International Conference on Coffee Science Colloquium, Montpellier, France, 11–15 September 2006; pp. 97–100. [Google Scholar]
- Stalmach, A.; Mullen, W.; Barron, D.; Uchida, K.; Yokota, T.; Cavin, C.; Steiling, H.; Williamson, G.; Crozier, A. Metabolite profiling of hydroxycinnamate derivatives in plasma and urine after the ingestion of coffee by humans: Identification of biomarkers of coffee consumption. Drug Metab. Dispos. 2009, 37, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahle, K.; Kempf, M.; Schreier, P.; Scheppach, W.; Schrenk, D.; Kautenburger, T.; Hecker, D.; Huemmer, W.; Ackermann, M.; Richling, E. Intestinal transit and systemic metabolism of apple polyphenols. Eur. J. Nutr. 2011, 50, 507–522. [Google Scholar] [CrossRef]
- Bermúdez-Soto, M.-J.; Tomás-Barberán, F.-A.; García-Conesa, M.-T. Stability of polyphenols in chokeberry (Aronia melanocarpa) subjected to in vitro gastric and pancreatic digestion. Food Chem. 2007, 102, 865–874. [Google Scholar] [CrossRef]
- Bouayed, J.; Deußer, H.; Hoffmann, L.; Bohn, T. Bioaccessible and dialysable polyphenols in selected apple varieties following in vitro digestion vs. their native patterns. Food Chem. 2012, 131, 1466–1472. [Google Scholar] [CrossRef]
- Ho, G.T.T.; Kase, E.T.; Wangensteen, H.; Barsett, H. Phenolic Elderberry extracts, anthocyanins, procyanidins, and metabolites influence glucose and fatty acid uptake in human skeletal muscle cells. J. Agric. Food Chem. 2017, 65, 2677–2685. [Google Scholar] [CrossRef] [PubMed]
- Posadino, A.M.; Cossu, A.; Giordo, R.; Piscopo, A.; Abdel-Rahman, W.M.; Piga, A.; Pintus, G. Antioxidant properties of olive mill wastewater polyphenolic extracts on human endothelial and vascular smooth muscle cells. Foods 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Goutzourelas, N.; Stagos, D.; Spanidis, Y.; Liosi, M.; Apostolou, A.; Priftis, A.; Haroutounian, S.; Spandidos, D.A.; Tsatsakis, A.M.; Kouretas, D. Polyphenolic composition of grape stem extracts affects antioxidant activity in endothelial and muscle cells. Mol. Med. Rep. 2015, 12, 5846–5856. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, Y.; Wang, Y.; Wen, Y.; Sun, C. Berberine improves free-fatty-acid-induced insulin resistance in L6 myotubes through inhibiting peroxisome proliferator-activated receptor γ and fatty acid transferase expressions. Metabolism. 2009, 58, 1694–1702. [Google Scholar] [CrossRef]
- Ommati, M.M.; Farshad, O.; Mousavi, K.; Khalili, M.; Jamshidzadeh, A.; Heidari, R. Chlorogenic acid supplementation improves skeletal muscle mitochondrial function in a rat model of resistance training. Biologia 2020, 75, 1221–1230. [Google Scholar] [CrossRef]
- Yun, N.; Kang, J.W.; Lee, S.M. Protective effects of chlorogenic acid against ischemia/reperfusion injury in rat liver: Molecular evidence of its antioxidant and anti-inflammatory properties. J. Nutr. Biochem. 2012, 23, 1249–1255. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Anti-diabetic and anti-lipidemic effects of chlorogenic acid are mediated by AMPK activation. Biochem. Pharmacol. 2013, 85, 1341–1351. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.W.; Hsu, A.; Tan, B.K.H. Chlorogenic acid stimulates glucose transport in skeletal muscle via AMPK activation: A contributor to the beneficial effects of coffee on diabetes. PLoS ONE 2012, 7, e32718. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid-induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Yang, H.; Jing, S.; Liu, B.; Wei, M.; He, P.; Zhang, N. Protective effect of chlorogenic acid on lipopolysaccharide-induced inflammatory response in dairy mammary epithelial cells. Microb. Pathog. 2018, 124, 178–182. [Google Scholar] [CrossRef]
- Shapses Sue, A.; Claudia, P. ; Wang Yang Obesity is a concern for bone health with aging. Nutr Res. 2017, 39, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zakłos-Szyda, M.; Nowak, A.; Pietrzyk, N.; Podsędek, A. Viburnum opulus L. juice phenolic compounds influence osteogenic differentiation in human osteosarcoma Saos-2 cells. Int. J. Mol. Sci. 2020, 21, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Flores, F.P.; Singh, R.K.; Kerr, W.L.; Pegg, R.B.; Kong, F. Total phenolics content and antioxidant capacities of microencapsulated blueberry anthocyanins during in vitro digestion. Food Chem. 2014, 153, 272–278. [Google Scholar] [CrossRef]
- Yang, I.F.; Jayaprakasha, G.K.; Patil, B.S. In vitro bile acid binding capacities of red leaf lettuce and cruciferous vegetables. J. Agric. Food Chem. 2017, 65, 8054–8062. [Google Scholar] [CrossRef]
- Miyazaki, J.I.; Araki, K.; Yamato, E.; Ikegami, H.; Asano, T.; Shibasaki, Y.; Oka, Y.; Yamamura, K.I.; Miyazaki, J.I. Establishment of a pancreatic β cell line that retains glucose-inducible insulin secretion: Special reference to expression of glucose transporter isoforms. Endocrinology 1990, 127, 126–132. [Google Scholar] [CrossRef]









| Peak | Compound | lmax | [M − H]− (m/z) | Sample | ||
|---|---|---|---|---|---|---|
| Undigested | OUT Fraction | IN Fraction | ||||
| Fresh juice (FJ) | ||||||
| 1 | Caffeoylquinic acid derivative I 1 | 337 | 353 | n.d. | 0.021 ± 0.008 | n.d. |
| 2 | Neochlorogenic acid | 323 | 353 | 0.007 ± 0.001 | 0.862 ± 0.067 | 0.582 ± 0.007 |
| 3 | Caffeoylquinic acid derivative II 1 | 323 | 707 | 0.015 ± 0.000 | n.d. | n.d. |
| 4 | Caffeoylquinic acid derivative III 1 | 323 | 707 | 0.024 ± 0.002 | n.d. | n.d. |
| 5 | Caffeoylquinic acid derivative IV 1 | 325 | 707 | 0.017 ± 0.001 | 0.173 ± 0.010 | 0.122 ± 0.015 |
| 6 | Chlorogenic acid | 324 | 353/707 | 8.039 ± 0.147 | 1.222 ± 0.129 | 0.612 ± 0.018 |
| 7 | Cryptochlorogenic acid | 325 | 353 | 0.004 ± 0.000 | 0.687 ± 0.087 | 0.435 ± 0.009 |
| 8 | Caffeoylquinic acid derivative V 1 | 325 | 705 | n.d. | 0.195 ± 0.059 | 0.091 ± 0.001 |
| 9 | Caffeoylquinic acid derivative VI 1 | 319 | 705 | n.d. | 0.166 ± 0.048 | 0.064 ± 0.001 |
| 10 | Caffeoylquinic acid | 313 | 353 | 0.745 ± 0.001 | 0.126 ± 0.049 | n.d. |
| 11 | NI | 323 | 531 | 0.017 ± 0.001 | 0.348 ± 0.059 | 0.259 ± 0.011 |
| 12 | Caffeoylquinic acid derivative VII 1 | 321 | 705 | 0.034 ± 0.000 | n.d. | n.d. |
| 13 | Caffeoylquinic acid derivative VIII 1 | 320 | 705 | 0.034 ± 0.000 | 0.178 ± 0.038 | 0.165 ± 0.012 |
| Purified juice (PJ) | ||||||
| 1 | Caffeoylquinic acid derivative I 1 | 337 | 353 | n.d. | 0.859 ± 0.050 | 0.070 ± 0.001 |
| 2 | Neochlorogenic acid | 322 | 353 | 0.215 ± 0.019 | 19.883 ± 0.295 | 4.141 ± 0.014 |
| 3 | Caffeoylquinic acid derivative II 1 | 323 | 707 | 1.289 ± 0.058 | n.d. | n.d. |
| 4 | Caffeoylquinic acid derivative III 1 | 323 | 707 | 1.051 ± 0.008 | n.d. | n.d. |
| 5 | Caffeoylquinic acid derivative IV 1 | 323 | 707 | 1.220 ± 0.020 | 1.367 ± 0.148 | 0.256 ± 0.002 |
| 6 | Chlorogenic acid | 318 | 353/707 | 645.492 ± 1.984 | 21.610 ± 0.040 | 3.330 ± 0.072 |
| 7 | Cryptochlorogenic acid | 323 | 353 | 0.484 ± 0.023 | 21.591 ± 0.072 | 5.902 ± 0.121 |
| 8 | Caffeoylquinic acid 1 | 313 | 353/707 | 44.344 ± 0.176 | 2.019 ± 0.467 | 0.328 ± 0.088 |
| 9 | Caffeoylquinic acid derivative V 1 | 325 | 705 | 3.306 ± 0.014 | 0.630 ± 0.065 | 0.324 ± 0.039 |
| 10 | Caffeoylquinic acid derivative VI 1 | 325 | 705 | 3.268 ± 0.010 | 0.839 ± 0.067 | 0.766 ± 0.034 |
| 11 | Feruloylquinic acid I 1 | 325 | 367 | 5.722 ± 0.021 | 1.387 ± 0.003 | 0.671 ± 0.064 |
| 12 | Feruloylquinic acid II 1 | 304 | 367 | 0.528 ± 0.005 | 1.716 ± 0.266 | n.d. |
Sample Availability: Samples of the compounds are not available from the authors. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pietrzyk, N.; Zakłos-Szyda, M.; Redzynia, M.; Podsędek, A. The Effect of Simulated In Vitro Digestion on Biological Activity of Viburnum opulus Fruit Juices. Molecules 2021, 26, 4086. https://doi.org/10.3390/molecules26134086
Pietrzyk N, Zakłos-Szyda M, Redzynia M, Podsędek A. The Effect of Simulated In Vitro Digestion on Biological Activity of Viburnum opulus Fruit Juices. Molecules. 2021; 26(13):4086. https://doi.org/10.3390/molecules26134086
Chicago/Turabian StylePietrzyk, Nina, Małgorzata Zakłos-Szyda, Małgorzata Redzynia, and Anna Podsędek. 2021. "The Effect of Simulated In Vitro Digestion on Biological Activity of Viburnum opulus Fruit Juices" Molecules 26, no. 13: 4086. https://doi.org/10.3390/molecules26134086
APA StylePietrzyk, N., Zakłos-Szyda, M., Redzynia, M., & Podsędek, A. (2021). The Effect of Simulated In Vitro Digestion on Biological Activity of Viburnum opulus Fruit Juices. Molecules, 26(13), 4086. https://doi.org/10.3390/molecules26134086