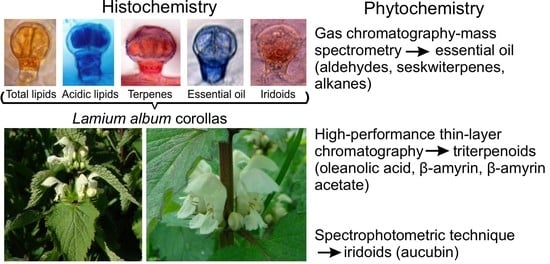
Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids
Abstract
:1. Introduction
2. Results
2.1. Morphometric Traits of Trichomes
2.1.1. Corolla
2.1.2. Stamen
2.2. Histochemistry
2.3. Phytochemical Analyses of the L. album subsp. album Corolla
2.3.1. Analysis of the Essential Oil Composition
2.3.2. High-Performance Thin Layer Chromatography (HPTLC) Analysis of Triterpenes
2.3.3. Spectrophotometric Analysis of Iridoids—Aucubin
3. Discussion
3.1. Trichomes
3.2. Essential Oil
3.3. Triterpenes
3.4. Iridoids
4. Materials and Methods
4.1. Plant Material
4.2. Light Microscopy—Handmade Preparations
4.3. Scanning Electron Microscopy (SEM) Preparations
4.4. Histochemical Analyses
4.5. Phytochemical Analyses
4.5.1. Essential Oil Isolation
4.5.2. Gas Chromatography-Mass Spectrometry (GC-MS)
4.5.3. Ethanolic Extract Preparation for Triterpene Analysis
4.5.4. Preparation of Organic Extracts for Identification of Triterpenes
4.5.5. Chromatographic High-Performance Thin Layer Chromatography (HPTLC) Analysis of Some Triterpenes
4.5.6. Selection of the Analytical Wavelength for Oleanolic Acid
4.5.7. Spectrophotometric—Quantitative Analysis of Iridoids
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Werker, E.; Putievsky, E.; Ravid, U.; Dudai, N.; Katzir, I. Glandular Hairs and Essential Oil in Developing Leaves of Ocimum basilicum L. (Lamiaceae). Ann. Bot. 1993, 71, 43–50. [Google Scholar] [CrossRef]
- Bergougnoux, V.; Caissard, J.C.; Jullien, F.; Magnard, J.L.; Scalliet, G.; Cock, J.M.; Baudino, S. Both the adaxial and abaxial epidermis layers of the rose petal emit volatile scent compounds. Planta 2007, 226, 853–866. [Google Scholar] [CrossRef]
- Vogel, S. The Role of Scent Glands in Pollination. On the Structure and Function of Osmophores; Amerind Publishing: New Delhi, India, 1990. [Google Scholar]
- Tölke, E.D.; Capelli, N.V.; Pastori, T.; Alencar, A.C.; Cole, T.C.H.; Demarco, D. Diversity of Floral Glands and Their Secretions in Pollinator Attraction. In Co-Evolution of Secondary Metabolites; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- Flamini, G.; Cioni, P.L.; Morelli, I. Composition of the essential oils and in vivo emission of volatiles of four Lamium species from Italy: L. purpureum, L. hybridum, L. bifidum and L. amplexicaule. Food Chem. 2005, 91, 63–68. [Google Scholar] [CrossRef]
- Madeira, S.V.F.; Rabelo, M.; Soares, P.M.G.; Souza, E.P.; Meireles, A.V.P.; Montenegro, C.; Lima, R.F.; Assreuy, A.M.S.; Criddle, D.N. Temporal variation of chemical composition and relaxant action of the essential oil of Ocimum gratissimum L. (Labiatae) on guinea-pig ileum. Phytomedicine 2005, 12, 506–509. [Google Scholar] [CrossRef]
- Barocelli, E.; Calcina, F.; Chiavarini, M.; Impicciatore, M.; Bruni, R.; Bianchi, A.; Ballabeni, V. Antinociceptive and gastroprotective effects of inhaled and orally administered Lavandula hybrida Reverchon “Grosso” essential oil. Life Sci. 2004, 76, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Król, S.K.; Kapka-Skrzypczak, L. Aktywność farmakologiczna olejków eterycznych i ich składników w leczeniu schorzeń układu pokarmowego. Med. Ogólna Nauk. Zdrowiu 2011, 17, 202–205. [Google Scholar]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1–120. [Google Scholar] [CrossRef]
- Schiestl, F.P. The evolution of floral scent and insect chemical communication. Ecol. Lett. 2010, 13, 643–656. [Google Scholar] [CrossRef]
- Farré-Armengol, G.; Filella, I.; Llusia, J.; Peñuelas, J. Floral volatile organic compounds: Between attraction and deterrence of visitors under global change. Perspect. Plant Ecol. Evol. Syst. 2013, 15, 56–67. [Google Scholar] [CrossRef]
- Antoń, S.; Kamińska, M.; Stpiczyńska, M. Comparative structure of the osmophores in the flower of Stanhopea graveolens Lindley and Cycnoches chlorochilon Klotzsch (Orchidaceae). Acta Agrobot. 2012, 65, 11–22. [Google Scholar] [CrossRef]
- Sulborska, A.; Dmitruk, M.; Konarska, A.; Weryszko-Chmielewska, E. Adaptations of Lamium album L. flowers to pollination by Apoidea. Acta Sci. Pol. Hortorum Cultus 2014, 13, 31–43. [Google Scholar]
- Bodnarčuk, L.I.; Solomacha, T.D.; Illjaš, A.M.; Solomacha, V.D.; Gorovyj, V.G. Atlas Medonosnych Roslyn Ukrainy; Urožaj: Kyiv, Ukraine, 1993; p. 268. [Google Scholar]
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020, 22, 961–970. [Google Scholar] [CrossRef] [PubMed]
- Cole, M.D. The Significance of Terpenoids in the Labiatae. In Advances in Labiatae Science; Harley, R.M., Reynolds, T., Eds.; Royal Botanic Gardens, Kew: Richmond, UK, 1992; pp. 315–324. [Google Scholar]
- Birkett, M.A.; Al Abassi, S.; Krober, T.; Chamberlain, K.; Hooper, A.M.; Guerin, P.M.; Petterson, J.; Pickett, J.A.; Slade, R.; Wadhams, L.J. Antiectoparasit activity of the gum resin, gum haggar, from the East Africa plant, Commiphora holtziana. Phytochemistry 2008, 69, 1710–1719. [Google Scholar] [CrossRef]
- Yordanova, Z.P.; Zhiponova, M.K.; Iakimova, E.T.; Dimitrova, M.A.; Kapchina-Toteva, V.M. Revealing the reviving secret of the white dead nettle (Lamium album L.). Phytochem. Rev. 2014, 13, 375–389. [Google Scholar] [CrossRef]
- Ghule, B.; Palve, S.; Rathi, L.; Yeole, P. Validated HPTLC method for simultaneous determination of shanzhiside methyl ester and barlerin in Barleria prionitis. JPC J. Planar Chromatogr. Mod. TLC 2012, 25, 426–432. [Google Scholar] [CrossRef]
- Cao, J.; Yu, H.; Wu, Y.; Wang, X. Occurrence and biological activities of phenylpropionyl iridoids. Mini Rev. Med. Chem. 2019, 19, 292–309. [Google Scholar] [CrossRef]
- Salehi, B.; Armstrong, L.; Rescigno, A.; Yeskaliyeva, B.; Seitimova, G.; Beyatli, A.; Sharifi-Rad, J. Lamium plants—A comprehensive review on health benefits and biological activities. Molecules 2018, 24, 1913. [Google Scholar] [CrossRef]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in terpene profiles of Thymus vulgaris in water deficit stress response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef]
- Holopainen, J.K.; Gershenzon, J. Multiple stress factors and the emission of plant VOCs. Trends Plant Sci. 2010, 15, 176–184. [Google Scholar] [CrossRef]
- Loreto, F.; Ciccioli, P.; Cecinato, A.; Brancaleoni, E.; Frattoni, M.; Tricoli, D. Influence of Environmental Factors and Air Composition on the Emission of [alpha]-Pinene from Quercus ilex Leaves. Plant Physiol. 1996, 110, 267–275. [Google Scholar] [CrossRef]
- Duhl, T.; Helmig, D.; Guenther, A. Sesquiterpene emissions from vegetation: A review. Biogeosciences 2008, 5, 761–777. [Google Scholar] [CrossRef]
- Morteza-Semnani, K.; Saeedi, M.; Akbarzadeh, M. Chemical composition of the essential oil of the flowering aerial parts of Lamium album L. J. Essent. Oil Bear. Plants 2016, 19, 773–777. [Google Scholar] [CrossRef]
- Mickiene, R.; Bakutis, B.; Maruska, A.; Ragazinskiene, O.; Kaskoniene, V. Effect of volatile secondary metabolites of Monarda didyma L., Lamium album L. and Myrrhis odorata L. Plants against micromycetes of indoor environments of animals. Veterinariia 2014, 68, 48–54. [Google Scholar]
- Kovalvoya, A.; Ilyina, T.; Kolesnik, Y. Study of component composition of the essential oil of leaves Lamium album. Pharmacology 2013, 1, 80–82. [Google Scholar]
- Alipieva, K.; Evstatieva, L.; Handjieva, N.; Popov, S. Comparative analysis of the composition of flower volatiles from Lamium L. species and Lamiastrum galeobdolon Heist. ex Fabr. Z. Nat. C 2003, 58, 779–782. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Urbanc, M.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic Implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Kiplimo, J.J.; Koorbanally, N.A.; Chenia, H. Triterpenoids from Vernonia auriculifera Hiern exhibit antimicrobial activity. Afr. J. Pharm. Pharmacol. 2011, 5, 1150–1156. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M. Antitumor and antiviral activity of pentacyclic triterpenes. Mini Rev. Org. Chem. 2014, 11, 262. [Google Scholar] [CrossRef]
- Ghiulai, R.; Roşca, O.J.; Antal, D.S.; Mioc, M.; Mioc, A.; Racoviceanu, R.; Şoica, C. Tetracyclic and Pentacyclic Triterpenes with High Therapeutic Efficiency in Wound Healing Approaches. Molecules 2020, 25, 5557. [Google Scholar] [CrossRef]
- Jian, T.; Ding, X.; Li, J.; Wu, Y.; Ren, B.; Li, J.; Lv, H.; Chen, J.; Li, W. Triterpene Acids of Loquat Leaf Improve Inflammation in Cigarette Smoking Induced COPD by Regulating AMPK/Nrf2 and NFκB Pathways. Nutrients 2020, 12, 657. [Google Scholar] [CrossRef] [PubMed]
- Wójciak-Kosior, M.; Krzaczek, T.; Matysik, G.; Skalska, A. HPTLC-densitometric method of determination of oleanolic acid in the Lamii albi flos. J. Sep. Sci. 2005, 28, 2139–2143. [Google Scholar] [CrossRef]
- Wójciak-Kosior, M.; Sowa, I.; Kocjan, R.; Nowak, R. Effect of different extraction techniques on quantification of oleanolic and ursolic acid in Lamii albi flos. Ind. Crop. Prod. 2013, 44, 373–377. [Google Scholar] [CrossRef]
- Paduch, R.; Wójciak-Kosior, M.; Matysik, G. Investigation of biological activity of Lamii albi flos extracts. J. Ethnopharmacol. 2007, 110, 69–75. [Google Scholar] [CrossRef]
- Kelayeh, T.P.S.; Abedinzade, M.; Ghorbani, A. A review on biological effects of Lamium album (white dead nettle) and its components. J. Herbmed. Pharmacol. 2019, 8, 185–193. [Google Scholar] [CrossRef]
- Eigtved, P.; Jensen, S.R.; Nielsen, B.J. A Novel Iridoid Glucoside Isolated from Lamium album L. Acta Chem. Scand. B 1974, 28, 85–91. [Google Scholar] [CrossRef]
- Damtoft, S. Iridoid glucosides from Lamium album. Phytochemistry 1992, 31, 175–178. [Google Scholar] [CrossRef]
- Yalcin, F.N.; Kaya, D. Ethnobotany, pharmacology and phytochemistry of the genus Lamium (Lamiaceae). FABAD J. Pharm. Sci. 2006, 31, 43–52. [Google Scholar]
- Budzianowski, J.; Skrzypczak, L. Phenylpropanoid esters from Lamium album flowers. Phytochemistry 1995, 38, 997–1001. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Taskova, R.M.; Evstatieva, L.N.; Handjieva, N.V.; Popov, S.S. Benzoxazinoids and iridoid glucosides from four Lamium species. Phytochemistry 2003, 64, 1413–1417. [Google Scholar] [CrossRef]
- Alipieva, K.I.; Taskova, R.M.; Jensen, S.R.; Handjieva, N.V. Iridoid glucosides from Lamium album and Lamium maculatum (Lamiaceae). Biochem. Syst. Ecol. 2006, 34, 88–91. [Google Scholar] [CrossRef]
- Alipieva, K.; Kokubun, T.; Taskova, R.; Evstatieva, L.; Handjieva, N. LC-ESI-MS analysis of iridoid glucosides in Lamium species. Biochem. Syst. Ecol. 2007, 35, 17–22. [Google Scholar] [CrossRef]
- Ersöz, T.; Kaya, D.; Yalcin, F.N.; Kazaz, C.; Palaska, E.; Gotfredsen, C.H.; Çaliş, İ. Iridoid glucosides from Lamium garganicum subsp. laevigatum. Turk. J. Chem. 2007, 31, 155–162. [Google Scholar]
- Adema, F. Iridoid glucosides of species of Lamium and some related genera. Acta Bot. Neerl. 1968, 17, 423–430. [Google Scholar] [CrossRef]
- Zhang, H.; Rothwangl, K.; Mesecar, A.D.; Sabahi, A.; Rong, L.; Fong, H.H. Lamiridosins, hepatitis C virus entry inhibitors from Lamium album. J. Nat. Prod. 2009, 72, 2158–2162. [Google Scholar] [CrossRef]
- Pashazadeh, M.; Rezaei, A. The Effect of Chornic Oral Lamium album Consumption on Blood Levels of Glucose and Lipids in Alloxan-Induced Diabetic Rats. Int. J. Pharm. Anal. 2013, 4, 21. [Google Scholar]
- Czerwińska, M.E.; Świerczewska, A.; Woźniak, M.; Kiss, A.K. Bioassay-Guided Isolation of Iridoids and Phenylpropanoids from Aerial Parts of Lamium album and Their Anti-inflammatory Activity in Human Neutrophils. Planta Med. 2017, 83, 1011–1019. [Google Scholar] [CrossRef]
- Matkowski, A.; Piotrowska, M. Antioxidant and free radical scavenging activities of some medicinal plants from the Lamiaceae. Fitoterapia 2006, 77, 346–353. [Google Scholar] [CrossRef]
- Available online: https://cosmetics.specialchem.com/inci/lamium-album-extract (accessed on 15 April 2021).
- Facciola, S. Cornucopia II: A Source Book of Edible Plants; Kampong Publications: Vista, CA, USA, 1998. [Google Scholar]
- Batsatsashvili, K.; Mehdiyeva, N.P.; Kikvidze, Z.; Khutsishvili, M.; Maisaia, I.; Sikharulidze, S.; Tchelidze, D.; Alizade, V.M.; Paniagua Zambrana, N.Y.; Bussmann, R.W. Lamium album L. Lamiaceae. In Ethnobotany of the Caucasus. European Ethnobotany; Bussmann, R., Ed.; Springer: Basel, Switerland, 2016. [Google Scholar] [CrossRef]
- Bartram, T. Bartram’s Encyclopedia of Herbal Medicine; Little Brown: London, UK, 1998. [Google Scholar]
- Łuczaj, Ł. Dzika Kuchnia. Wyd; Nasza Księgarnia: Warszawa, Polska, 2013. [Google Scholar]
- Hatfield, G. Hatfield’s Herbal: The Curious Stories of Britain’s Wild Plants; Penguin Books: London, UK, 2007. [Google Scholar]
- Sulborska, A.; Konarska, A.; Matysik-Woźniak, A.; Dmitruk, M.; Weryszko-Chmielewska, E.; Skalska-Kamińska, A.; Rejdak, R. Phenolic Constituents of Lamium album L. subsp. album Flowers: Anatomical, Histochemical, and Phytochemical Study. Molecules 2020, 25, 6025. [Google Scholar] [CrossRef]
- Celep, F.; Kahraman, A.; Atalay, Z.; Dogan, M. Morphology, anatomy and trichome properties of Lamium truncatum Boiss. (Lamiaceae) and their systematic implications. Aust. J. Crop Sci. 2011, 5, 147–153. [Google Scholar]
- Özdemir, C.; Baran, P. Morphological, anatomical and cytological investigation on alpine Lamium cymbalariifolium endemic to Turkey. Aust. J. Crop Sci. 2012, 6, 532–540. [Google Scholar]
- Atalay, Z.; Celep, F.; Bara, F.; Doğan, M. Systematic significance of anatomy and trichome morphology in Lamium (Lamioideae; Lamiaceae). Flora 2016, 225, 60–75. [Google Scholar] [CrossRef]
- Zvezdina, E.V.; Dayronas, J.V.; Bochkareva, I.I.; Zilfikarov, I.N.; Babaeva, E.Y.; Ferubko, E.V.; Ibragimov, T.A. Members of the family Lamiaceae Lindl. as sources of medicinal plant raw materials to obtain neurotropic drugs. Pharm. Pharmacol. 2020, 8, 4–28. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectroscopy, 4th ed.; Allured Pub Corp.: Carol Stream, IL, USA, 2007. [Google Scholar]
- Sharifi-Rad, J.; Sureda, A.; Tenore, G.C.; Daglia, M.; Sharifi-Rad, M.; Valussi, M.; Tundis, R.; Sharifi-Rad, M.; Loizzo, M.R.; Ademiluyi, A.O.; et al. Biological Activities of Essential Oils: From Plant Chemoecology to Traditional Healing Systems. Molecules 2017, 22, 70. [Google Scholar] [CrossRef]
- Huang, S.S.; Kirchoff, B.K.; Liao, J.P. The capitate and peltate glandular trichomes in Lavandula pinnata L. (Lamiaceae): Histochemistry, ultrastructure and secretion. J. Torrey Bot. Soc. 2008, 135, 155–167. [Google Scholar] [CrossRef]
- Jia, P.; Gao, T.; Xin, H. Changes in structure and histochemistry of glandular trichomes of Thymus quinquecostatus Celak. Sci. World J. 2012, 187261. [Google Scholar] [CrossRef]
- Giuliani, C.; Bini, L.M. Insight into the structure and chemistry of glandular trichomes of Labiatae, with emphasis on subfamily Lamioideae. Plant Syst. Evol. 2008, 276, 199–208. [Google Scholar] [CrossRef]
- Naidoo, Y.; Kasim, N.; Heneidak, S.; Nicholas, A.; Naidoo, G. Foliar secretory trichomes of Ocimum obovatum (Lamiaceae): Micromorphological structure and histochemistry. Plant Syst. Evol. 2013, 299, 873–885. [Google Scholar] [CrossRef]
- Dmitruk, M.; Sulborska, A.; Żuraw, B.; Stawiarz, E.; Weryszko-Chmielewska, E. Sites of secretion of bioactive compounds in leaves of Dracocephalum moldavica L.: Anatomical, histochemical, and essential oil study. Braz. J. Bot. 2019, 42, 701–715. [Google Scholar] [CrossRef]
- Ascensão, L.; Mota, L.; Castro, M.D.M. Glandular Trichomes on the Leaves and Flowers of Plectranthus ornatus: Morphology, Distribution and Histochemistry. Ann. Bot. 1999, 84, 437–447. [Google Scholar] [CrossRef]
- Corsi, G. Glandular Hairs of Salvia officinalis: New Data on Morphology, Localization and Histochemistry in Relation to Function. Ann. Bot. 1999, 84, 657–664. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Damiano, S.; Papa, F.; Vittori, S.; Bini, L.M.; Giuliani, C.; et al. Phytochemical Analysis, Biological Activity, and Secretory Structures of Stachys annua (L.) L. subsp. annua (Lamiaceae) from Central Italy. Chem. Biodivers. 2015, 12, 1172–1183. [Google Scholar] [CrossRef]
- Venditti, A.; Bianco, A.; Frezza, C.; Conti, F.; Bini, L.M.; Giuliani, C.; Bramucci, M.; Quassinti, L.; Damiano, S.; Lupidi, G.; et al. Essential oil composition, polar compounds, glandular trichomes and biological activity of Hyssopus officinalis subsp. aristatus (Godr.) Nyman from central Italy. Ind. Crop. Prod. 2015, 77, 253–363. [Google Scholar] [CrossRef]
- Combrinck, S.; Du Plooy, G.W.; McCrindle, R.I.; Botha, B.M. Morphology and Histochemistry of the Glandular Trichomes of Lippia scaberrima (Verbenaceae). Ann. Bot. 2007, 99, 1111–1119. [Google Scholar] [CrossRef]
- Sacchetti, G.; Romagnoli, C.; Nicoletti, M.; Di Fabio, A.; Bruni, A.; Poli, F. Glandular trichomes of Calceolaria adscendens Lidl. (Scrophulariaceae): Histochemistry, development and ultrastructure. Ann. Bot. 1999, 83, 87–92. [Google Scholar] [CrossRef]
- Ventrella, M.C.; Marinho, C.R. Morphology and histochemistry of glandular trichomes of Cordia verbenacea DC. (Boraginaceae) leaves. Br. J. Bot. 2008, 31, 457–467. [Google Scholar] [CrossRef]
- Haratym, W.; Weryszko-Chmielewska, E. Ultrastructural and histochemical analysis of glandular trichomes of Marrubium vulgare L. (Lamiaceae). Flora 2017, 231, 11–20. [Google Scholar] [CrossRef]
- Evert, R.F. Esau’s Plant Anatomy, 3rd ed.; Wiley-Interscience: Hoboken, NJ, USA, 2006. [Google Scholar]
- Hejnowicz, Z. Anatomia i Histogeneza Roślin Naczyniowych; Wydawnictwo Naukowe PWN: Warszawa, Poland, 2018. [Google Scholar]
- Tozin, L.R.S.; Silva, C.M.; Rodrigues, T.M. Non-glandular trichomes in Lamiaceae and Verbenaceae species: Morphological and histochemical features indicate more than physical protection. N. Z. J. Bot. 2016, 54, 446–457. [Google Scholar] [CrossRef]
- Asekun, O.T.; Grierson, D.S.; Afolayan, A.J. Effects of drying methods on the quality and quantity of the essential oil of Mentha longifolia L. subsp. capensis. Food Chem. 2007, 101, 995–998. [Google Scholar] [CrossRef]
- Figiel, A.; Szumny, A.; Gutiérrez-Ortíz, A.; Carbonell-Barrachina, Á.A. Composition of oregano essential oil (Origanum vulgare) as affected by drying method. J. Food Eng. 2010, 98, 240–247. [Google Scholar] [CrossRef]
- Turek, C.; Stintzing, F.C. Stability of essential oils: A review. Compr. Rev. Food Sci. Food Saf. 2013, 12, 40–53. [Google Scholar] [CrossRef]
- Lewicki, P.P.; Pawlak, G. Effect of drying on microstructure of plant tissue. Dry.Technol. 2003, 12, 657–683. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Astatkie, T.; Zhalnov, I.; Georgieva, T.D. Method for attaining rosemary essential oil with differential composition from dried or fresh material. J. Oleo Sci. 2015, 64, ess14258. [Google Scholar] [CrossRef]
- Mashkani, M.R.D.; Larijani, K.; Mehrafarin, A.; Badi, H.N. Changes in the essential oil content and composition of Thymus daenensis Celak. under different drying methods. Ind. Crop. Prod. 2018, 112, 389–395. [Google Scholar] [CrossRef]
- Silva, S.G.; da Costa, R.A.; de Oliveira, M.S.; da Cruz, J.N.; Figueiredo, P.L.B.; Brasil, D.D.S.B.; Andrade, E.H.D.A. Chemical profile of Lippia thymoides, evaluation of the acetylcholinesterase inhibitory activity of its essential oil, and molecular docking and molecular dynamics simulations. PLoS ONE 2019, 14, e0213393. [Google Scholar] [CrossRef]
- Kapchina-Toteva, V.; Dimitrova, M.A.; Stefanova, M.; Koleva, D.; Kostov, K.; Yordanova, Z.P.; Stefanov, D.; Zhiponova, M.K. Adaptive changes in photosynthetic performance and secondary metabolites during white dead nettle micropropagation. J. Plant Physiol. 2014, 171, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Pudziuvelyte, L.; Stankevicius, M.; Maruska, A.; Petrikaite, V.; Ragazinskiene, O.; Draksiene, G.; Bernatoniene, J. Chemical composition and anticancer activity of Elsholtzia ciliata essential oils and extracts prepared by different methods. Ind. Crop. Prod. 2017, 107, 90–96. [Google Scholar] [CrossRef]
- Beigi, M.; Torki-Harchegani, M.; Ghasemi Pirbalouti, A. Quantity and chemical composition of essential oil of peppermint (Mentha × piperita L.) leaves under different drying methods. Int. J. Food Prop. 2018, 21, 267–276. [Google Scholar] [CrossRef]
- Raffo, A.; Mozzanini, E.; Nicoli, S.F.; Lupotto, E.; Cervelli, C. Effect of light intensity and water availability on plant growth, essential oil production and composition in Rosmarinus officinalis L. Eur. Food Res. Technol. 2020, 246, 167–177. [Google Scholar] [CrossRef]
- Castelo, A.V.M.; Del Menezzi, C.H.S.; Resck, I.S. Seasonal variation in the yield and the chemical composition of essential oils from two Brazilian natives arbustive species. J. Appl. Sci. 2012, 12, 753–760. [Google Scholar] [CrossRef]
- Pljevljakušić, D.; Janković, T.; Jelacić, S.; Novaković, M.; Menković, N.; Beatović, D.; Dajić-Stevanović, Z. Morphological and chemical characterization of Arnica montana L. under different cultivation models. Ind. Crop. Prod. 2014, 52, 233–244. [Google Scholar] [CrossRef]
- Avci, A.B.; Giachino, R.R.A. Harvest stage effects on some yield and quality characteristics of lemon balm (Melissa officinalis L.). Ind. Crop. Prod. 2016, 88, 23–27. [Google Scholar] [CrossRef]
- Shams, M.; Ramezani, M.; Esfahan, S.Z.; Esfahan, E.Z.; Dursun, A.; Yildirim, E. Effects of climatic factors on the quantity of essential oil and dry matter yield of coriander (Coriandrum sativum L.). J. Sci. Technol. 2016, 9, 1–4. [Google Scholar] [CrossRef]
- Tawfeeq, A.; Culham, A.; Davis, F.; Reeves, M. Does fertilizer type and method of application cause significant differences in essential oil yield and composition in rosemary (Rosmarinus officinalis L.)? Ind. Crop. Prod. 2016, 88, 17–22. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Romero, M.J.; Llanderal, A.; Cermeño, P.; Lao, M.T.; Segura, M.L. Effects of drought stress on biomass, essential oil content, nutritional parameters, and costs of production in six Lamiaceae species. Water 2019, 11, 573. [Google Scholar] [CrossRef]
- Salman, M.; Abdel-Hameed, E.S.S.; Bazaid, S.A.; Dadi, M.M. Chemical composition for hydrodistillation essential oil of Mentha longifolia by gas chromatography-mass spectrometry from north regions in kingdom of Saudi Arabia. Pharm. Chem. 2015, 7, 34–40. [Google Scholar]
- Sharopov, F.S.; Wink, M.; Khalifaev, D.R.; Zhang, H.; Dosoky, N.S.; Setzer, W.N. Composition and bioactivity of the essential oil of Melissa officinalis L. growing wild in Tajikistan. Int. J. Tradit. Nat. Med. 2013, 2, 86–96. [Google Scholar]
- Martins, T.G.T.; Rosa, P.V.S.; Arruda, M.O.; Dias, A.A.S.; de Araújo Neto, A.P.; Carvalho, A.M.A.S.; Everton, G.O. Larvicidal activity of microparticles of Melissa officinalis L. essential oil (Lamiaceae) against Aedes aegypti (Diptera, Culicidae). Res. Soc. Dev. 2021, 10, e35710111166. [Google Scholar] [CrossRef]
- Petrović, S.; Ušjak, L.; Milenković, M.; Arsenijević, J.; Drobac, M.; Drndarević, A.; Niketić, M. Thymus dacicus as a new source of antioxidant and antimicrobial metabolites. J. Funct. Foods 2017, 28, 114–121. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Deans, S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000, 88, 308–316. [Google Scholar] [CrossRef]
- Chizzola, R. Regular monoterpenes and sesquiterpenes (essential oils). In Natural Products; Ramawat, K.G., Mérillon, J.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 2973–3008. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Orcic, D.; Lesjak, M.; Sibul, F. Essential oils as powerful antioxidants: Misconception or scientific fact? In Medicinal and Aromatic Crops: Production, Phytochemistry and Utilization; American Chemical Society: Washington, DC, USA, 2016; pp. 187–208. [Google Scholar] [CrossRef]
- Nickavar, B.; Mojab, F.; Bamasian, S. Volatile components from aerial parts of Lamium amplexicaule from Iran. J. Essent. Oil Bear. Plants 2008, 11, 36–40. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, Y.J.; Bao, J.; Huang, L.; Nielsen, J.; Krivoruchko, A. Metabolic engineering of Saccharomyces cerevisiae for production of germacrene A, a precursor of beta-elemene. J. Ind. Microb. Biotechnol. 2017, 44, 1065–1072. [Google Scholar] [CrossRef]
- Wang, Y.; Jiang, Z.T.; Li, R. Antioxidant Activity, Free Radical Scavenging Potential and Chemical Composition of Litsea cubeba Essential Oil. J. Essent. Oil Bear. Plants 2012, 15, 134–143. [Google Scholar] [CrossRef]
- Casigliaa, S.; Brunoa, M.; Bramuccib, M.; Quassintib, L.; Lupidic, D.; Fiorinic, D.; Maggi, F. Kundmannia sicula (L.) DC: A rich source of germacrene D. J. Essent. Oil Res. 2017, 29, 437–442. [Google Scholar] [CrossRef]
- El-Sayed, Z.I.A. Chemical composition, antimicrobial and insecticidal activities of the essential oil of Lamium maculatum L. grown in Egypt. Biosci. Biotechnol. Res. Asia 2008, 5, 65–72. [Google Scholar]
- Yang, D.; Michel, L.; Chaumont, J.P.; Millet-Clerc, J. Use of caryophyllene oxide as an antifungal agent in an in vitro experimental model of onychomycosis. Mycopathologia 2000, 148, 79–82. [Google Scholar] [CrossRef] [PubMed]
- Oh, M.S.; Yang, J.Y.; Kim, M.G.; Lee, H.S. Acaricidal activities of β-caryophyllene oxide and structural analogues derived from Psidium cattleianum oil against house dust mites. Pest Manag. Sci. 2014, 70, 757–762. [Google Scholar] [CrossRef]
- Nararak, J.; Sathantriphop, S.; Kongmee, M.; Mahiou-Leddet, V.; Ollivier, E.; Manguin, S.; Chareonviriyaphap, T. Excito-repellent activity of β-caryophyllene oxide against Aedes aegypti and Anopheles minimus. Acta Trop. 2019, 197, 105030. [Google Scholar] [CrossRef]
- Park, K.-R.; Nam, D.; Yun, H.-M.; Lee, S.-G.; Jang, H.-J.; Sethi, G.; Cho, S.K.; Ahn, K.S. β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/Akt/mTOR/S6K1 pathways and ROS-mediated mapks activation. Cancer Lett. 2011, 312, 178–188. [Google Scholar] [CrossRef]
- Dahham, S.S.; Tabana, Y.M.; Iqbal, M.A.; Ahamed, M.B.; Ezzat, M.O.; Majid, A.S.; Majid, A.M. The Anticancer, Antioxidant and Antimicrobial Properties of the Sesquiterpene β-Caryophyllene from the Essential Oil of Aquilaria crassna. Molecules 2015, 20, 11808–11829. [Google Scholar] [CrossRef]
- Fidyt, K.; Fiedorowicz, A.; Strządała, L.; Szumny, A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016, 10, 3007–3017. [Google Scholar] [CrossRef]
- Myszka, K.; Schmidt, M.T.; Majcher, M.; Juzwa, W.; Czaczyk, K. β-Caryophyllene-rich pepper essential oils suppress spoilage activity of Pseudomonas fluorescens KM06 in fresh-cut lettuce. LWT Food Sci. Technol. 2017, 83, 118–126. [Google Scholar] [CrossRef]
- Jones, C.D.; Woods, K.E.; Setzer, W.N. A chemical ecological investigation of the allelopathic potential of Lamium amplexicaule and Lamium purpureum. Open J. Ecol. 2012, 2, 167–177. [Google Scholar] [CrossRef]
- Wang, L.; Well, C.L. Recent advances in extraction of nutraceuticals from plants. Trends Food Sci. Technol. 2006, 17, 300–312. [Google Scholar] [CrossRef]
- Baker, D.A. Plants against Helicobacter pylori to combat resistance: An ethnopharmacological review. Biotechnol. Rep. 2020, 26, e00470. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, A.; Victoria Awolola, G.; Ibrahim, M.A.; Anthony Koorbanally, N.; Islam, M.S. Oleanolic acid as a potential antidiabetic component of Xylopia aethiopica (Dunal) A. Rich. (Annonaceae) fruit: Bioassay guided isolation and molecular docking studies. Nat. Prod. Res. 2021, 35, 788–791. [Google Scholar] [CrossRef]
- Fernandes, C.P.; Corrêa, A.L.; Lobo, J.F.R.; Caramel, O.P.; de Almeida, F.B.; Castro, E.S.; Souza, K.F.C.S.; Burth, P.; Amorim, L.M.F.; Santos, M.G.; et al. Triterpene Esters and Biological Activities from Edible Fruits of Manilkara subsericea (Mart.) Dubard, Sapotaceae. BioMed Res. Int. 2013, 280810. [Google Scholar] [CrossRef]
- Okoye, N.N.; Ajaghaku, D.L.; Okeke, H.N.; Ilodigwe, E.E.; Nworu, C.S.; Okoye, F.B.C. Beta-Amyrin and alpha-amyrin acetate isolated from the stem bark of Alstonia boonei display profound anti-inflammatory activity. Pharm. Biol. 2014, 52, 1478–1486. [Google Scholar] [CrossRef]
- De Almeida, P.D.O.; de Boleti, A.P.; Rüdiger, A.L.; Lourenço, G.A.; Florêncio da Veiga Junior, V.; Lima, E.S. Anti-Inflammatory Activity of Triterpenes Isolated from Protium paniculatum Oil-Resins. Evid. Based Complement. Altern. Med. 2015, 293768. [Google Scholar] [CrossRef]
- Henneh, I.T.; Huang, B.; Musayev, F.N.; Al Hashimi, R.; Safo, M.K.; Armah, F.A.; Ameyaw, E.O.; Adokoh, C.K.; Ekor, M.; Zhang, Y. Structural elucidation and in vivo anti-arthritic activity of β-amyrin and polpunonic acid isolated from the root bark of Ziziphus abyssinica Hochst Ex. A Rich (Rhamnaceae). Biorgan. Chem. 2020, 98, 103744. [Google Scholar] [CrossRef]
- El-Desouky, S.K.; Abdelgawad, A.A.; El-Hagrassi, A.M.; Hawas, U.W.; Kim, Y.-K. Chemical composition, cytotoxic and antioxidant activities of Celosia trigyna L. grown in Saudi Arabia. Acta Pol. Pharm. Drug Res. 2019, 76, 691–699. [Google Scholar] [CrossRef]
- Abdel Bar, F.M.; Abbas, G.M.; Gohar, A.A.; Lahloub, M.-F.I. Antiproliferative activity of stilbene derivatives and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2020, 34, 3506–3513. [Google Scholar] [CrossRef] [PubMed]
- Damtoft, S.; Jensen, S.R.; Nielsen, B.J. Biosynthesis of iridoid glucosides in Lamium album. Phytochemistry 1992, 31, 135–137. [Google Scholar] [CrossRef]
- Czerwińska, M.E.; Świerczewska, A.; Granica, S. Bioactive constituents of Lamium album L. as inhibitors of cytokine secretion in human neutrophils. Molecules 2018, 23, 2770. [Google Scholar] [CrossRef] [PubMed]
- Czerwińska, M.E.; Kalinowska, E.; Popowski, D.; Bazylko, A. Lamalbid, chlorogenic acid and verbascoside as tools for standardization of Lamium album flowers-development and validation of HPLC–DAD method. Molecules 2020, 25, 1721. [Google Scholar] [CrossRef]
- Zhang, S.; Feng, Z.; Gao, W.; Duan, Y.; Fan, G.; Geng, X.; Wu, B.; Li, K.; Liu, K.; Peng, C. Aucubin Attenuates Liver Ischemia-Reperfusion Injury by Inhibiting the HMGB1/TLR-4/NF-kB Signaling Pathway, Oxidative Stress, and Apoptosis. Front. Pharmacol. 2020, 11, 544124. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Duan, J.-X.; Liu, T.; Guan, X.-X.; Mei, W.-X.; Liu, Y.-P.; Sun, G.-Y.; Wan, L.; Zhong, W.-J.; et al. Aucubin Alleviates Bleomycin-Induced Pulmonary Fibrosis in a Mouse Model. Inflammation 2017, 40, 2062–2073. [Google Scholar] [CrossRef]
- Dąbrowska, M.; Nowak, I. Noninvasive evaluation of the influence of aucubin-containing cosmetic macroemulsion on selected skin parameters. J. Cosmet. Dermatol. 2021, 20, 1022–1030. [Google Scholar] [CrossRef]
- Hegi, G. Illustrierte Flora von Mittel-Europa; Carl Hanser: München, Germany, 1965. [Google Scholar]
- Mennema, J. A Taxonomic Revision of Lamium (Lamiaceae); Brill Archive: Paderborn, Germany, 1989; Volume 11. [Google Scholar]
- Stpiczyńska, M.; Davies, K.L.; Pacek-Bieniek, A.; Kamińska, M. Comparative anatomy of the floral elaiophore in representatives of the newly re-circumscribed Gomesa and Oncidium clades (Orchidaceae: Oncidiinae). Ann. Bot. 2013, 112, 839–854. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique, 1st ed.; McGraw Hill: New York, NY, USA; London, UK, 1940. [Google Scholar]
- Pearse, A. Pigment and pigment precursors. In Histochemistry, Theoretical and Applied; Pearse, A., Ed.; Churchhill Livingstone: London, UK, 1985; Volume 2, pp. 874–928. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with Sudan Red 7 B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Lison, L. Histochemie et Cytochemie Animals. Principes et Mèthods; Gauthier-Villars: Paris, France, 1960; Volume 1. [Google Scholar]
- Cain, A.J. The use of Nile blue in the examination of lipids. J. Cell Sci. 1947, 88, 383–392. [Google Scholar] [CrossRef]
- Jensen, W.A. Botanical Histochemistry Principles and Practice, 1st ed.; WH Freeman and Company: San Francisco, CA, USA, 1962. [Google Scholar]
- David, R.; Carde, J.P. Coloration différentielle des inclusions lipidique et terpéniques des pseudophylles du pin maritime au moyen du réactif Nadi. CR Acad. Sci. Paris 1964, 258, 1338–1340. [Google Scholar]
- Kirk, P.W., Jr. Neutral red as a lipid fluorochrome. Stain Technol. 1970, 45, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Clark, G. Staining Procedures, 4th ed.; Williams and Wilkins: Baltimore, MD, USA, 1981. [Google Scholar]
- Inouye, H. Iridoids. In Methods in Biochemistry; Charlwood, B.V., Banthorpe, D.V., Eds.; Academic Press: London, UK, 1991; Volume 7, pp. 99–143. [Google Scholar]
- Polish Pharmaceutical Society. Polish Pharmacopoeia, 6th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 2002. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromat. 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy, 3th ed.; Allured Pub Corp.: Carol Stream, IL, USA, 2001. [Google Scholar]
- Mass Spectral Library. NIST/EPA/NIH, USA. 2020. Available online: https://chemdata.nist.gov/dokuwiki/doku.php?id=chemdata:start (accessed on 26 May 2020).
- Polish Pharmaceutical Society. Polish Pharmacopoeia, 5th ed.; Polish Pharmaceutical Society: Warsaw, Poland, 1999. [Google Scholar]
- Strzelecka, H.; Kamińska, J.; Kowalski, J.; Malinowski, J.; Walewska, E. Chemiczne Metody Badań Roślinnych Surowców Leczniczych; PZWL: Warszawa, Polska, 1987. [Google Scholar]













| Types of Trichomes | Height (μm) | Diameter (μm) * | |||
|---|---|---|---|---|---|
| Min–Max | Mean | Min–Max | Mean | ||
| Corolla | |||||
| Short-stalked glandular trichomes | |||||
| Peltate | 26.0–31.2 | 29.5 ± 1.8 | 71.3–97.6 | 80.8 ± 10.5 | |
| Capitate with 1-celled head | 23.4–29.0 | 25.5 ± 2.0 | 28.6–40.6 | 35.4 ± 5.3 | |
| Capitate with 2- and 3-celled head | 23.4–31.7 | 27.9 ± 2.8 | 28.6–52.8 | 43.0 ± 8.6 | |
| Capitate with 4-celled head | 26.4–31.7 | 29.5 ± 1.8 | 34.3–44.9 | 39.7 ± 0.0 | |
| Non-glandular trichomes | |||||
| Long 1-celled | 102.3–204.6 | 161.6 ± 34.5 | 10.2–20.5 | 15.1 ± 3.6 | |
| Long 2–4 celled | 409.0–1273.8 | 753.2 ± 330.8 | 12.3–25.6 | 17.9 ± 5.0 | |
| Conical | 40.9–197.6 | 125.2 ± 55.8 | 21.5–72.8 | 41.7 ± 18.9 | |
| Flattened long 1-celled | 409.2–480.8 | 437.8 ± 28.5 | 25.6–51.2 | 35.8 ± 9.2 | |
| Stamen | |||||
| Long-stalked glandular trichomes | |||||
| Capitate with 1–4 celled head | 15.8–28.6 | 20.9 ± 4.2 | 97.6–213.0 | 157.1 ± 39.5 | |
| Non-glandular trichomes | |||||
| Long 1 celled | 613.8–945.6 | 778.6 ± 110.5 | 10.2–18.5 | 14.6 ± 2.8 | |
| Test | Compound | Colour Observed | Corolla | Stamens | |||
|---|---|---|---|---|---|---|---|
| Papillae | Types of Trichomes | ||||||
| Short Capitate | Peltate | Non Glandular | Long Capitate | ||||
| Sudan Black B | total lipids | dark blue | + | + | + | + | nd |
| Sudan Red B | total lipids | red | + | + | ++ | + | ++ |
| Sudan III | total lipids | orange | + | ++ | ++ | ± | + |
| Nile Blue | acidic lipids | blue | ++ | ++ | ++ | ++ | ++ |
| neutral lipids | pink | − | − | − | − | − | |
| Nadi reagent | terpenes, essential oil | violet-blue or purple | + | ++ | ++ | ± | ± |
| Neutral Red | terpenoids, essential oil | red | + | ++ | ++ | + | + |
| Godin reagent | iridoids | violet or pink | ± | + | + | ++ | nd |
| No. | Compound | Retention Time (min) | RRI | AI | Dry Corollas % | Fresh Corollas % |
| Monoterpenes | ||||||
| Monoterpenes hydrocarbon | ||||||
| 1 | α-thujene | 9.79 | 932 | 924 | 0.7 | - |
| 2 | camphene | 10.32 | 946 | 946 | 0.1 | 0.2 |
| 3 | limonene + β-phellandrene | 13.40 | 1029 | - | 0.4 | - |
| 4 | γ-terpinene | 14.55 | 1060 | 1062 | 0.2 | - |
| Oxygenated monoterpenes | ||||||
| 1 | 2,3-dehydro-1,8-cineole | 11.89 | 989 | 988 | - | 0.6 |
| 2 | 2-pentyl-furan | 12.03 | 993 | 984 | 0.5 | 0.2 |
| 3 | terpinen-4-ol | 18.95 | 1178 | 1174 | 0.1 | - |
| 4 | isogeranial | 19.23 | 1186 | 1160 | - | 0.2 |
| 5 | β-citronellol | 20.84 | 1232 | 1223 | - | 0.3 |
| 6 | carvone | 21.29 | 1244 | 1239 | 1.4 | |
| 7 | geranyl acetate | 26.07 | 1386 | 1379 | - | 0.4 |
| Monoterpenoids | ||||||
| Oxygenated | ||||||
| 1 | α-terpineol | 19.44 | 1192 | 1186 | - | 0.4 |
| Sesquiterpenes | ||||||
| Sesquiterpenes hydrocarbon | ||||||
| 1 | δ-elemene | 24.53 | 1339 | 1335 | 1.4 | |
| 2 | longicyclene | 25.47 | 1378 | 1371 | - | 0.4 |
| 3 | α-copaene | 25.82 | 1378 | 1374 | 0.46 | 0.1 |
| 4 | β-elemene | 26.33 | 1394 | 1398 | 2.7 | 0.1 |
| 4 | (E)-β-caryophyllene | 27.22 | 1422 | 1417 | 6.6 | 0.4 |
| 5 | germacrene D | 29.17 | 1484 | 1484 | 21.2 | - |
| 6 | γ-cadinene | 30.19 | 1517 | 1513 | 1.1 | 2.7 |
| 7 | δ-cadinene | 30.46 | 1526 | 1522 | 0.4 | 0.2 |
| Oxygenated sesquiterpenes | ||||||
| 1 | caryophyllene oxide | 32.25 | 1587 | 1582 | 8.2 | 12.5 |
| Sesquiterpenoids | ||||||
| Sesquiterpenoids hydrocarbon | ||||||
| 1 | β-bourbonene | 26.10 | 1387 | 1387 | 0.6 | - |
| 2 | (E)-α-bergamotene | 27.73 | 1438 | 1434 | - | 0.3 |
| Oxygenated sesquiterpenoids | ||||||
| 1 | epi-cubebol | 29.59 | 1497 | 1493 | - | 0.2 |
| 2 | 10-epi-cubebol | 30.68 | 1534 | 1533 | 0.4 | 0.6 |
| 3 | epi-cubenol isomer | 33.18 | 1619 | - | 0.2 | 0.3 |
| 4 | γ-eudesmol | 33.65 | 1635 | 1630 | - | 0.3 |
| 5 | epi-α-cadinol | 33.96 | 1646 | 1638 | - | 0.3 |
| 6 | α-eudesmol | 34.20 | 1655 | 1652 | 0.1 | 0.2 |
| Alkybenzenes | ||||||
| 1 | p-cymene | 13.27 | 1026 | 1089 | 0.1 | - |
| Aldehydes | ||||||
| Aromatic | ||||||
| 1 | benzeneacetaldehyde | 14.30 | 1053 | - | 0.2 | - |
| Aliphatic unsaturated aldehydes | ||||||
| 1 | isoneral | 18.47 | 1165 | 1160 | - | 0.3 |
| 2 | neral | 21.18 | 1241 | 1235 | 4.3 | 23.2 |
| 3 | geranial | 22.21 | 1271 | 1264 | 8.4 | 36.4 |
| Alcohols | ||||||
| Unsaturated aliphatic alcohols | ||||||
| 1 | linalool | 16.08 | 1100 | 1098 | 0.5 | 0.7 |
| 2 | nonen-1-ol | 16.23 | 1104 | 1152 | 0.7 | - |
| 3 | elemol | 31.22 | 1552 | 1548 | 1.6 | 2.2 |
| Cyclic alcohols | ||||||
| 1 | borneol | 18.47 | 1165 | 1165 | - | 0.2 |
| 2 | cedrol | 32.79 | 1605 | 1600 | - | 1.7 |
| Alkanes | ||||||
| 1 | n-tetradecane | 26.56 | 1401 | 1400 | 0.1 | - |
| 2 | branched-chain alkane | 28.51 | 1463 | - | 21.9 | - |
| Ketones | ||||||
| 1 | piperitone | 21.68 | 1256 | 1249 | - | 0.4 |
| 2 | (E)-β-damascenone | 26.10 | 1387 | 1361 | 0.6 | - |
| Epoxides | ||||||
| 1. | 1,2-humulene epoxide | 33.02 | 1613 | 1608 | 0.9 | 0.9 |
| Unknown | ||||||
| 1 | unknown 1284 | 22.49 | 1279 | - | - | 1.4 |
| 2 | unknown 1291 | 22.67 | 1284 | - | - | 2.0 |
| 3 | unknown 1679 | 34.76 | 1675 | - | - | 0.9 |
| * Total 91.1% | * Total 85.6% | |||||
| Monoterpenes hydrocarbon | ||||||||
 |  |  |  |  | ||||
| thujene | camphene | limonene | β-phellandrene | γ-terpinene | ||||
| Oxygenated monoterpenes | ||||||||
 |  |  |  |  |  |  | ||
| 2,3-dehydro-1,8-cineole | 2-pentyl-furan | terpinen-4-ol | isogeranial | β-citronellol | carvone | geranyl acetate | ||
| Monoterpenoids oxygenated | ||||||||
 | ||||||||
| α-terpineol | ||||||||
| Sesquiterpenes hydrocarbon | ||||||||
 |  |  |  |  | ||||
| δ-elemene | longicyclene | α-copaene | β-elemene | (E)-β-caryophyllene | ||||
 |  |  | ||||||
| germacrene D | γ-cadinene | δ-cadinene | ||||||
| Oxygenated sesquiterpenes | Sesquiterpenoids hydrocarbon | |||||||
 |  |  | ||||||
| caryophyllene oxide | β-bourbonene | (E)-α-bergamotene | ||||||
| Oxygenated sesquiterpenoids | ||||||||
 |  |  |  | |||||
| 10-epi-cubebol | γ-eudesmol | α-eudesmol | epi-α-cadinol | |||||
| Alkybenzenes | Aromatic aldehydes | Aliphatic unsaturated aldehydes | ||||||
 |  |  |  |  | ||||
| p-cymene | benzene acetaldehyde | isoneral | neral | geranial | ||||
| Unsaturated aliphatic alcohols | Cyclic alcohols | |||||||
 |  |  |  |  | ||||
| linalool | 3-nonen-1-ol | elemol | borneol | cedrol | ||||
| Alkanes | Ketones | Epoxides | ||||||
 |  |  |  | |||||
| n-tetradecane | piperitone | (E)-β-damascenone | 1,2-humulene epoxide | |||||
| Chemical Structure | |||
|---|---|---|---|
| Triterpenes | Iridoids | ||
| β-amyrin | β-amyrin acetate | oleanolic acid | aucubin |
 |  |  |  |
| Extract Type | Iridoid Average Content Expressed as Aucubin (mg/mL) | Variance s2 | Standard Deviation s | sr | Confidence Interval 95% |
|---|---|---|---|---|---|
| Lamium album flowers | 0.0015 | 1.0 × 10−8 | 1.0 × 10−4 | 1.6 | 0.0015 ± 4.3 × 10−4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konarska, A.; Weryszko-Chmielewska, E.; Matysik-Woźniak, A.; Sulborska, A.; Polak, B.; Dmitruk, M.; Piotrowska-Weryszko, K.; Stefańczyk, B.; Rejdak, R. Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids. Molecules 2021, 26, 4166. https://doi.org/10.3390/molecules26144166
Konarska A, Weryszko-Chmielewska E, Matysik-Woźniak A, Sulborska A, Polak B, Dmitruk M, Piotrowska-Weryszko K, Stefańczyk B, Rejdak R. Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids. Molecules. 2021; 26(14):4166. https://doi.org/10.3390/molecules26144166
Chicago/Turabian StyleKonarska, Agata, Elżbieta Weryszko-Chmielewska, Anna Matysik-Woźniak, Aneta Sulborska, Beata Polak, Marta Dmitruk, Krystyna Piotrowska-Weryszko, Beata Stefańczyk, and Robert Rejdak. 2021. "Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids" Molecules 26, no. 14: 4166. https://doi.org/10.3390/molecules26144166
APA StyleKonarska, A., Weryszko-Chmielewska, E., Matysik-Woźniak, A., Sulborska, A., Polak, B., Dmitruk, M., Piotrowska-Weryszko, K., Stefańczyk, B., & Rejdak, R. (2021). Histochemical and Phytochemical Analysis of Lamium album subsp. album L. Corolla: Essential Oil, Triterpenes, and Iridoids. Molecules, 26(14), 4166. https://doi.org/10.3390/molecules26144166