Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization
Abstract
:1. Introduction

2. Results and Discussions
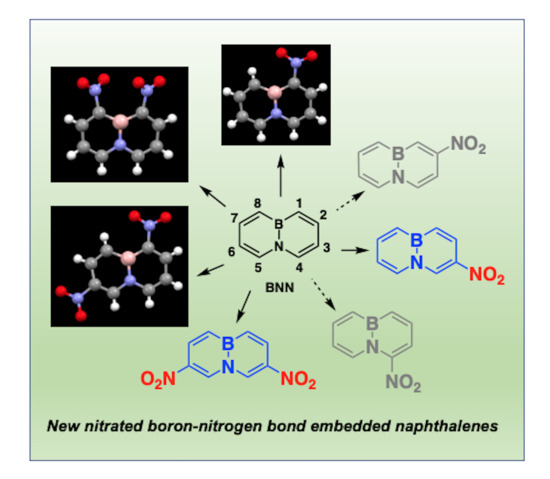
2.1. Nitration of BN-Naphthalene

2.2. The Spectral and Structural Features of Nitro-BNNs
3. Conclusions
4. Experimental Section
4.1. Nitration of BNN with AcONO2
4.2. Nitration of BNN with NO2BF4
4.3. Nitration of BNN with One Equivalent of AcONO2 in CH3CN
4.4. Nitration of BNN with One Equivalent of NO2BF4 in CH3CN
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Yang, D.-T.; Zheng, J.; Peng, J.-B.; Wang, X.; Wang, S. Sequential and Diverse Synthesis of BN-Heterocycles and Investigation of Their Photoreactivity. J. Org. Chem. 2021, 86, 829–836. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, W.; Qiao, Y.; Lu, X.; Zhou, G. BN-Embedded Polycyclic Aromatic Hydrocarbon Oligomers: Synthesis, Aromaticity, and Reactivity. Angew. Chem. Int. Ed. 2020, 59, 7122–7130. [Google Scholar] [CrossRef] [PubMed]
- Abengozar, A.; Sucunza, D.; Garcia-Garcia, P.; Perez-Redondo, A.; Vaquero, J.J.; Sampedro, D.A. New Member of the BN-Phenanthrene Family: Understanding the Role of the B-N Bond Position. J. Org. Chem. 2019, 84, 7113–7122. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, F.-D.; Sun, Z.-H.; Yao, Z.-F.; Chen, Q.-R.; Huang, Z.; Yang, J.-H.; Wang, J.-Y.; Pei, J. BN-Embedded Tetrabenzopentacene: A Pentacene Derivative with Improved Stability. Angew. Chem. Int. Ed. 2019, 58, 10708–10712. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw Heidi, L.; Klausen, R.S. BN Polystyrenes: Emerging Optical Materials and Versatile Intermediates. J. Org. Chem. 2019, 84, 1117–1125. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Z.; Li, Z.; Xu, Y.; Liang, J.; Lin, C.; Wei, J.; Wang, Y. Achieving Efficient Blue Delayed Electrofluorescence by Shielding Acceptors with Carbazole Units. ACS Appl. Mater. Interfaces 2019, 11, 28096–28105. [Google Scholar] [CrossRef] [PubMed]
- Fukagawa, H.; Oono, T.; Iwasaki, Y.; Hatakeyama, T.; Shimizu, T. High-efficiency ultrapure green organic light-emitting diodes. Mater. Chem. Front. 2018, 2, 704–709. [Google Scholar] [CrossRef]
- Yoshida, K.; Osuka, A. Pyrrole-Modified Subporphyrins Bearing a Sulfur-Containing Heterocyclic Unit. Helv. Chim. Acta 2018, 101, e1800025. [Google Scholar] [CrossRef]
- Min, Y.; Dou, C.; Tian, H.; Geng, Y.; Liu, J.; Wang, L. n-Type Azaacenes Containing B→N Units. Angew. Chem. Int. Ed. 2018, 57, 2000–2004. [Google Scholar] [CrossRef]
- Wang, T.; Dou, C.; Liu, J.; Wang, L. Effects of the Substitutes of Boron Atoms on Conjugated Polymers Containing B←N Units. Chem. Eur. J. 2018, 24, 13043–13048. [Google Scholar] [CrossRef]
- Cassidy, S.J.; Brettell-Adams, I.; McNamara, L.E.; Smith, M.F.; Bautista, M.; Cao, H.; Vasiliu, M.; Gerlach, D.L.; Qu, F.; Hammer, N.I.; et al. Boranes with Ultra-High Stocks Shift Fluorescence. Organometallics 2018, 37, 3732–3741. [Google Scholar] [CrossRef]
- Wang, X.-Y.; Wang, J.-Y.; Pei, J. BN Heterosuperbenzenes: Synthesis and Properties. Chem. Eur. J. 2015, 21, 3528–3539. [Google Scholar] [CrossRef]
- Min, Y.; Cao, X.; Tian, H.; Liu, J.; Wang, L. B←N Incorporated Dibenzoazaacene with Selective Near-Infrared Absorption and Visible Transparency. Chem. Eur. J. 2021, 27, 2065–2071. [Google Scholar] [CrossRef]
- Zhao, R.; Min, Y.; Dou, C.; Lin, B.; Ma, W.; Liu, J.; Wang, L. A Conjugated Polymer Containing a B←N Unit for Unipolar n-Type Organic Field-Effect Transistors. ACS Appl. Polym. Mater. 2020, 2, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, X.H.; Younis, U.; Qie, Y.; Aftab, W.; Sun, Q. A BN analog of two-dimensional triphenylene-graphdiyne: Stability and properties. Nanoscale 2019, 11, 9000–9007. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, J.S.A.; Darrigan, C.; Chrostowska, A.; Li, B.; Liu, S.-Y. A BN anthracene mimics the electronic structure of more highly conjugated systems. Dalton Trans. 2019, 48, 2807–2812. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, T.; Hashimoto, S.; Seki, S.; Nakamura, M. Synthesis of BN-fused Polycyclic Aromatics via Tandem Intramolecular Electrophilic Arene Borylation. J. Am. Chem. Soc. 2011, 133, 18614–18617. [Google Scholar] [CrossRef] [PubMed]
- Palomino-Ruiz, L.; Rodriguez-Gonzalez, S.; Fallaque, J.G.; Marquez, I.R.; Agrait, N.; Diaz, C.; Leary, E.; Cuerva, J.M.; Campana, A.G.; Martin, F.; et al. Single-Molecule Conductance of 1,4-Azaborine Derivatives as Models of BN-doped PAHs. Angew. Chem. Int. Ed. 2021, 60, 6609–6616. [Google Scholar] [CrossRef]
- Maar, R.R.; Katzman, B.D.; Boyle, P.D.; Staroverov, V.N.; Gilroy, J.B. Cationic boron formazanate dyes. Angew. Chem. Int. Ed. 2021, 60, 5152–5156. [Google Scholar] [CrossRef]
- Chen, M.; Unikela, K.S.; Ramalakshmi, R.; Li, B.; Darrigan, C.; Chrostowska, A.; Liu, S.-Y. A BN-doped cycloparaphenylene debuts. Angew. Chem. Int. Ed. 2021, 60, 1556–1560. [Google Scholar] [CrossRef]
- Zhang, Q.; Sun, Z.; Zhang, L.; Li, M.; Zi, L.; Liu, Z.; Zhen, B.; Sun, W.; Liu, X. Synthesis, Structures, and Properties of BN-Dinaphthothiophenes: Influence of B and N Placement on Photophysical Properties and Aromaticity. J. Org. Chem. 2020, 85, 7877–7883. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.D.; Wang, J.Y.; Pei, J. Recent Progress and Challenges in the Synthesis of Boron-nitrogen Fused Ploycyclic Aromatic Hydrocarbons. Sci. Sin. Chim. 2020, 50, 1205–1216. [Google Scholar]
- Tsuchiya, S.; Saito, H.; Nogi, K.; Yorimitsu, H. Aromatic Metamorphosis of Indoles into 1,2-Benzazaborins. Org. Lett. 2019, 21, 3855–3860. [Google Scholar] [CrossRef]
- Baranac-Stojanovic, M. A DFT Study of the Modulation of the Antiaromatic and Open-Shell Character of Dibenzo[a,f]pentalene by Employing Three Strategies: Additional Benzoannulation, BN/CC Isosterism, and Substitution. Chemistry 2019, 25, 9747–9757. [Google Scholar] [CrossRef]
- Anafcheh, M.; Ahmadi, E.; Zahedi, M. Addition of borazine to boron nitride nanotubes: [2+2] cycloaddition or bond cleavage. Mon. Chem. 2019, 150, 1019–1024. [Google Scholar] [CrossRef]
- van de Wouw, H.L.; Awuyah, E.C.; Baris, J.I.; Klausen, R.S. An Organoborane Vinyl Monomer with Styrene-like Radical Reactivity: Reactivity Ratios and Role of Aromaticity. Macromolecules 2018, 51, 6359–6368. [Google Scholar] [CrossRef]
- Zhu, C.; Ji, X.; You, D.; Chen, T.L.; Mu, A.U.; Barker, K.P.; Klivansky, L.M.; Liu, Y.; Fang, L. Extraordinary Redox Activities in Ladder-Type Conjugated Molecules Enabled by B←N Coordination-Promoted Delocalization and Hyperconjugation. J. Am. Chem. Soc. 2018, 140, 18173–18182. [Google Scholar] [CrossRef] [PubMed]
- Abengózar, A.; Fernández-González, M.A.; Sucunza, D.; Frutos, L.M.; Salgado, A.; García-García, P.; Vaquero, J.J. C−H Functionalization of BN-Aromatics Promoted by Addition of Organolithium Compounds to the Boron Atom. Org. Lett. 2018, 20, 4902–4906. [Google Scholar] [CrossRef] [PubMed]
- Schraff, S.; Sun, Y.; Pammer, F. Tuning of Electronic Properties Vialabile N-B-coordiation in Conjugated Organoboranes. J. Mater. Chem. C 2017, 5, 1730–1741. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Kubba, V.P. New heteroaromatic compounds. IV. Nitration and chlorination of 10-methyl-10,9-borazarophenanthrene. Tetrahedron 1959, 7, 213–222. [Google Scholar] [CrossRef]
- Zhang, Y.; Dan, W.; Fang, X. Metal nitrate mediated regioselective nitration of BN-substituted arenes. Organometallics 2017, 36, 1677–1680. [Google Scholar] [CrossRef]
- Dewar, M.J.S.; Gleicher, G.J.; Robinson, B.P. Synthesis and Nuclear Magnetic Resonance Spectrum of 10,9-Borazanaphthalene. J. Am. Chem. Soc. 1964, 86, 5698–5699. [Google Scholar] [CrossRef]
- Olah, G.; Narang, S.; Olah, J.; Lammertsma, K. Recent aspects of nitration: New preparative methods and mechanistic studies (a review). Proc. Nat. Acad. Sci. USA 1982, 79, 4487–4494. [Google Scholar] [CrossRef] [Green Version]
- Olah, G.A.; Narang, S.C.; Olah, J.A. Nitration of naphthalene and remarks on the mechanism of electrophilic aromatic nitration. Proc. Nat. Acad. Sci. USA 1981, 78, 3298–3300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, F.A.; Dewar, M.J.S.; Jones, R. New heterocyclic compounds. XXVII. Boron-11 chemical shifts of some heterocyclic boron compounds. J. Am. Chem. Soc. 1968, 31, 706–708. [Google Scholar] [CrossRef]
- Hermanek, S. B-11 NMR spectra of boranes, main-group heteroboranes, and substituted derivatives. Factors influencing chemical shifts of skeletal atoms. Chem. Rev. 1992, 92, 324–362. [Google Scholar] [CrossRef]
- Michl, J. Borazaro analogues of aromatic hydrocarbons. II. Semiempirical calculations. Collect. Czechoslov. Chem. Commun. 1971, 36, 1248–1278. [Google Scholar] [CrossRef]
- Baranac-Stojanovic, M.; Stojanovic, M. Does aromaticity account for an enhanced thermodynamic stability? The case of monosubstituted azaborines and the stereoelectronic chameleonism of the NH2 group. Phys. Chem. Phys. 2019, 21, 9465–9476. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Yang, H.; Kampf, J.W.; Banaszak, H.; Mark, M.; Ashe, A.J., III. Synthesis of Ring-Fused B-N Heterocyclic Compounds. Organometallics 2006, 25, 513–518. [Google Scholar] [CrossRef]
- Pejlovas, A.M.; Daly, A.M.; Ashe, A.J., III; Kukolich, S.G. Microwave spectra, molecular structure, and aromatic character of 4a,8a-azaboranaphthalene. J. Chem. Phys. 2016, 144, 114303–114310. [Google Scholar] [CrossRef]
- Stojanovic, M.; Baranac-Stojanovic, M. Mono BN-substituted analogues of naphthalene: A theoretical analysis of the effect of BN position on stability, aromaticity, and frontier orbital energies. New J. Chem. 2018, 42, 12968–12976. [Google Scholar] [CrossRef] [Green Version]
- Krygowski, T.M.; Szatylowicz, H.; Stasyuk, O.A.; Dominikowska, J.; Palusiak, M. Aromaticity from the viewpoint of molecular geometry: Application to planar systems. Chem. Rev. 2014, 114, 6383–6422. [Google Scholar] [CrossRef] [PubMed]


| BNNs |  |  |  |  |  |
|---|---|---|---|---|---|
| 11B NMR (ppm) | 29.9 | 29.5 | 27.8 | 25.6 | 22.6 |
| Boron q | 0.518 | 0.524 | 0.548 | 0.580 | 0.624 |

| Bond Type and Bond Length (Å) a | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| BC1 | C1C2 | C2C3 | C3C4 | C4N | BN | C5N | C5C6 | C6C7 | C7C8 | BC8 | |
| BNN b BNN c | 1.455 1.510 | 1.357 1.352 | 1.431 1.435 | 1.360 1.384 | 1.445 1.391 | 1.461 1.470 | 1.445 1.391 | 1.361 1.384 | 1.431 1.435 | 1.357 1.352 | 1.455 1.510 |
| 4 | 1.525 | 1.358 | 1.415 | 1.359 | 1.382 | 1.472 | 1.395 | 1.343 | 1.427 | 1.364 | 1.510 |
| 6 | 1.517 | 1.368 | 1.412 | 1.344 | 1.395 | 1.469 | 1.381 | 1.353 | 1.423 | 1.350 | 1.520 |
| 7 | 1.520 | 1.356 | 1.396 | 1.337 | 1.387 | 1.460 | 1.387 | 1.337 | 1.396 | 1.356 | 1.520 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, M.-X.; Zuckerman, N.B.; Pagoria, P.F.; Steele, B.A.; Kuo, I.-F.; Imler, G.H.; Parrish, D. Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization. Molecules 2021, 26, 4209. https://doi.org/10.3390/molecules26144209
Zhang M-X, Zuckerman NB, Pagoria PF, Steele BA, Kuo I-F, Imler GH, Parrish D. Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization. Molecules. 2021; 26(14):4209. https://doi.org/10.3390/molecules26144209
Chicago/Turabian StyleZhang, Mao-Xi, Nathaniel B. Zuckerman, Philip F. Pagoria, Bradley A. Steele, I-Feng Kuo, Gregory H. Imler, and Damon Parrish. 2021. "Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization" Molecules 26, no. 14: 4209. https://doi.org/10.3390/molecules26144209
APA StyleZhang, M. -X., Zuckerman, N. B., Pagoria, P. F., Steele, B. A., Kuo, I. -F., Imler, G. H., & Parrish, D. (2021). Mono- and Dinitro-BN-Naphthalenes: Formation and Characterization. Molecules, 26(14), 4209. https://doi.org/10.3390/molecules26144209