Flexo-Ionic Effect of Ionic Liquid Crystal Elastomers
Abstract
:1. Introduction
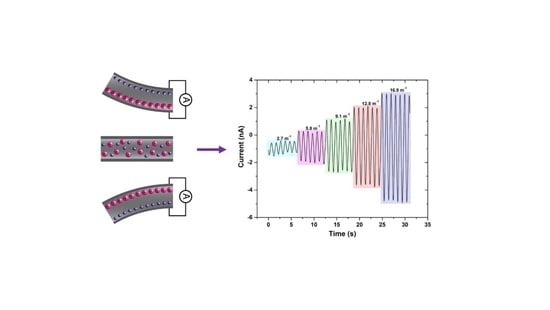
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Sample Preparations
3.3. Experimental Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Feng, C.; Rajapaksha, C.P.H.; Cedillo, J.M.; Piedrahita, C.; Cao, J.; Kaphle, V.; Lussem, B.; Kyu, T.; Jákli, A.I. Electro-Responsive Ionic Liquid Crystal Elastomers. Macromol. Rapid Commun. 2019, 40, 1900299. [Google Scholar] [CrossRef]
- Rajapaksha, C.P.H.; Feng, C.; Piedrahita, C.; Cao, J.; Kaphle, V.; Lüssem, B.; Kyu, T.; Jákli, A. Poly(Ethylene Glycol) Diacrylate Based Electro-Active Ionic Elastomer. Macromol. Rapid Commun. 2020, 41, 1900636. [Google Scholar] [CrossRef] [PubMed]
- Kim, O.; Kim, S.J.; Park, M.J. Low-Voltage-Driven Soft Actuators. Chem. Commun. 2018, 54, 4895–4904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, C.; Hemantha Rajapaksha, C.P.; Jákli, A. Ionic Elastomers for Electric Actuators and Sensors. Engineering 2021, 7, 581–602. [Google Scholar] [CrossRef]
- Wu, J.M.; Chen, C.Y.; Zhang, Y.; Chen, K.H.; Yang, Y.; Hu, Y.; He, H.; Wang, Z.L. Ultrahigh Sensitive Piezotronic Strain Sensors Based on a ZnSnO3 Nanowire/Microwire. ACS Nano 2012, 6, 4369–4374. [Google Scholar] [CrossRef]
- Piedrahita, C.R.; Yue, P.; Cao, J.; Lee, H.; Rajapaksha, C.P.; Feng, C.; Jákli, A.; Kyu, T. Flexoelectricity in Flexoionic Polymer Electrolyte Membranes: Effect of Thiosiloxane Modification on Poly (Ethylene Glycol) Diacrylate and Ionic Liquid Electrolyte Composites. ACS Appl. Mater. Interfaces 2020, 12, 16978–16986. [Google Scholar] [CrossRef] [PubMed]
- Albehaijan, H.A.; Piedrahita, C.R.; Cao, J.; Soliman, M.; Mitra, S.; Kyu, T. Mechanoelectrical Transduction of Polymer Electrolyte Membranes: Effect of Branched Networks. ACS Appl. Mater. Interfaces 2020, 12, 7518–7528. [Google Scholar] [CrossRef]
- Albehaijan, H.A.; Cao, J.; Piedrahita, C.R.; Jákli, A.; Kyu, T. Role of Cationic Size and Valency in Mechanoelectrical Transduction of Ion-Containing Polymers. ACS Sustain. Chem. Eng. 2021, 9, 1837–1845. [Google Scholar] [CrossRef]
- Cao, J.; Piedrahita, C.; Kyu, T. Mechanoelectrical Conversion in Highly Ionic Conductive Solid-State Polymer Electrolyte Membranes. Macromol. Mater. Eng. 2019, 304, 1800777. [Google Scholar] [CrossRef]
- Ikeda, T. Piezoelectricity and Pyroelectricity. In Fundamentals of Piezoelectricity; Oxford University Press: New York, NY, USA, 1990; pp. 1–30. [Google Scholar]
- Mayer, R.M. Piezoelectric Effects in Liquid Crystals. Phys. Rev. Lett. 1969, 22, 25–29. [Google Scholar] [CrossRef]
- Ma, W.; Cross, L.E. Flexoelectric Effect in Ceramic Lead Zirconate Titanate. Appl. Phys. Lett. 2005, 86, 1–3. [Google Scholar] [CrossRef]
- Ma, W. Flexoelectricity: Strain Gradient Effects in Ferroelectrics. Phys. Scr. 2007, T129, 180–185. [Google Scholar] [CrossRef]
- Chu, B.; Salem, D.R. Flexoelectricity in Several Thermoplastic and Thermosetting Polymers. Appl. Phys. Lett. 2012, 101, 103905. [Google Scholar] [CrossRef]
- Marvan, M.; Havránek, A. Flexoelectric Effect in Elastomers. In Relationships of Polymeric Structure and Properties; Steinkopff: Darmstadt, Germany, 2007; Volume 36, pp. 33–36. [Google Scholar]
- Schmidt, D.; Schadt, M.; Helfrich, W. Liquid-Crystalline Curvature Electricity: The Bending Mode of MBBA. Z. Nat. A 1972, 26, 277–280. [Google Scholar] [CrossRef]
- Buka, Á.; Éber, N. Flexoelectricity in Liquid Crystals, 1st ed.; Buka, A., Eber, N., Eds.; Imperial College Press: London, UK, 2013. [Google Scholar]
- Harden, J.; Mbanga, B.; Iber, N.; Fodor-Csorba, K.; Sprunt, S.; Gleeson, J.T.; Jákli, A. Giant Flexoelectricity of Bent-Core Nematic Liquid Crystals. Phys. Rev. Lett. 2006, 97, 13–16. [Google Scholar] [CrossRef] [Green Version]
- Harden, J.; Chambers, M.; Verduzco, R.; Luchette, P.; Gleeson, J.T.; Sprunt, S.; Jákli, A. Giant Flexoelectricity in Bent-Core Nematic Liquid Crystal Elastomers. Appl. Phys. Lett. 2010, 96, 102907. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Kim, H.; Choi, U.H.; Park, M.J. One-Volt-Driven Superfast Polymer Actuators Based on Single-Ion Conductors. Nat. Commun. 2016, 7, 13576–13584. [Google Scholar] [CrossRef] [Green Version]
- Kim, O.; Kim, S.Y.; Park, B.; Hwang, W.; Park, M.J. Factors Affecting Electromechanical Properties of Ionic Polymer Actuators Based on Ionic Liquid-Containing Sulfonated Block Copolymers. Macromolecules 2014, 47, 4357–4368. [Google Scholar] [CrossRef]
- Kim, O.; Shin, T.J.; Park, M.J. Fast Low-Voltage Electroactive Actuators Using Nanostructured Polymer Electrolytes. Nat. Commun. 2013, 4, 3208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Aida, T. “Bucky Gels” for Tailoring Electroactive Materials and Devices: The Composites of Carbon Materials with Ionic Liquids. Chem. Commun. 2011, 47, 6757–6762. [Google Scholar] [CrossRef]
- Bahramzadeh, Y.; Shahinpoor, M. A Review of Ionic Polymeric Soft Actuators and Sensors. Soft Robot. 2013, 1, 38–52. [Google Scholar] [CrossRef]
- Wei, D.; Ivaska, A. Applications of Ionic Liquids in Electrochemical Sensors. Anal. Chim. Acta 2008, 607, 126–135. [Google Scholar] [CrossRef]
- Cao, J.; Rendon Piedrahita, C.; Zhao, Z.; Vogt, B.D.; Kyu, T. Tuning Flexoelectric Effect in Polymer Electrolyte Membranes via Cation Selection for Potential Energy Harvesting Applications. ACS Appl. Energy Mater. 2020, 3, 328–335. [Google Scholar] [CrossRef]
- Margaretta, E.; Fahs, G.B.; Inglefield, D.L.; Jangu, C.; Wang, D.; Heflin, J.R.; Moore, R.B.; Long, T.E. Imidazolium-Containing ABA Triblock Copolymers as Electroactive Devices. ACS Appl. Mater. Interfaces 2016, 8, 1280–1288. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Wang, D.; Heflin, J.R.; Long, T.E. Imidazolium Sulfonate-Containing Pentablock Copolymer-Ionic Liquid Membranes for Electroactive Actuators. J. Mater. Chem. 2012, 22, 13473–13476. [Google Scholar] [CrossRef]
- Bar-Cohen, Y. Electroactive Polymer (EAP) Actuators as Artificial Muscles: Reality, Potential, and Challenges; SPIE Press: Bellingham, WA, USA, 2004; Volume 136. [Google Scholar]
- Takeuchi, I.; Asaka, K.; Kiyohara, K.; Sugino, T.; Terasawa, N.; Mukai, K.; Fukushima, T.; Aida, T. Electromechanical Behavior of Fully Plastic Actuators Based on Bucky Gel Containing Various Internal Ionic Liquids. Electrochim. Acta 2009, 54, 1762–1768. [Google Scholar] [CrossRef]
- Imaizumi, S.; Kato, Y.; Kokubo, H.; Watanabe, M. Driving Mechanisms of Ionic Polymer Actuators Having Electric Double Layer Capacitor Structures. J. Phys. Chem. B 2012, 116, 5080–5089. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, T.; Asaka, K.; Kosaka, A.; Aida, T. Fully Plastic Actuator through Layer-by-Layer Casting with Ionic-Liquid-Based Bucky Gel. Angew. Chem. Int. Ed. 2005, 44, 2410–2413. [Google Scholar] [CrossRef]
- Asaka, K.; Mukai, K.; Sugino, T.; Kiyohara, K.; Takeuchi, I.; Terasawa, N.; Aida, T.; Futaba, D.N.; Hata, K.; Fukushima, T. Highly Conductive Sheets from Millimeter-Long Single-Walled Carbon Nanotubes and Ionic Liquids: Application to Fast-Moving, Low-Voltage Electromechanical Actuators Operable in Air. Adv. Mater. 2009, 21, 1582–1585. [Google Scholar] [CrossRef]
- Jangu, C.; Wang, J.H.H.; Wang, D.; Fahs, G.; Heflin, J.R.; Moore, R.B.; Colby, R.H.; Long, T.E. Imidazole-Containing Triblock Copolymers with a Synergy of Ether and Imidazolium Sites. J. Mater. Chem. C 2015, 3, 3891–3901. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.W.; Yu, S.; Hong, S.M.; Koo, C.M. High-Strain Air-Working Soft Transducers Produced from Nanostructured Block Copolymer Ionomer/Silicate/Ionic Liquid Nanocomposite Membranes. J. Mater. Chem. C 2013, 1, 3784–3793. [Google Scholar] [CrossRef]
- Green, M.D.; Wang, D.; Hemp, S.T.; Choi, J.H.; Winey, K.I.; Heflin, J.R.; Long, T.E. Synthesis of Imidazolium ABA Triblock Copolymers for Electromechanical Transducers. Polymer 2012, 53, 3677–3686. [Google Scholar] [CrossRef]
- Binder, K. Collective Diffusion, Nucleation, and Spinodal Decomposition in Polymer Mixtures. J. Chem. Phys. 1983, 79, 6387–6409. [Google Scholar] [CrossRef]
- Kaphle, V.; Liu, S.; Al-Shadeedi, A.; Keum, C.-M.; Lüssem, B. Contact Resistance Effects in Highly Doped Organic Electrochemical Transistors. Adv. Mater. 2016, 28, 8766–8770. [Google Scholar] [CrossRef]
- Cao, J. Bioelectricity Inspired Polymer Electrolyte Membranes for Sensing and Engergy Harvesting Applications. Ph.D. Thesis, University of Akron, Akron, OH, USA, 2018. [Google Scholar]
- Kwon, S.R.; Huang, W.; Shu, L.; Yuan, F.G.; Maria, J.P.; Jiang, X. Flexoelectricity in Barium Strontium Titanate Thin Film. Appl. Phys. Lett. 2014, 105, 142904. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liu, J.; Hu, X.; Chu, B.; Chen, S.; Salem, D. Flexoelectric Effect in PVDF-Based Polymers. IEEE Trans. Dielectr. Electr. Insul. 2017, 24, 727–731. [Google Scholar] [CrossRef]






| Materials | Flexoelectric/Ionic Coefficient | Driving Frequencies |
|---|---|---|
| iLCEs (This study) | 25–220 | 0.01–1 Hz |
| PEM (TS-PEGDA/IL) | 84–154 [6] | 0.01–10 Hz |
| PEM (PEGDA/IL) | 53–125 [6] | 0.01–1 Hz |
| PEM (PEGDA/SCN/LiTFSI) | 29–323 [9] | 0.01 Hz |
| bent-core liquid crystal | 60 [18] | 1–10 Hz |
| Bent-core liquid crystal elastomer | 30 [19] | 0.2–12 Hz |
| PVDF | 2–13 [41] | 6 Hz |
| Pb [ZrxTi1−x] O3 (PZT) | 1.4 [12] | 1 Hz |
| Ba0.67Sr0.33TiO3 | 100 [40] | 2 Hz |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajapaksha, C.P.H.; Gunathilaka, M.D.T.; Narute, S.; Albehaijan, H.; Piedrahita, C.; Paudel, P.; Feng, C.; Lüssem, B.; Kyu, T.; Jákli, A. Flexo-Ionic Effect of Ionic Liquid Crystal Elastomers. Molecules 2021, 26, 4234. https://doi.org/10.3390/molecules26144234
Rajapaksha CPH, Gunathilaka MDT, Narute S, Albehaijan H, Piedrahita C, Paudel P, Feng C, Lüssem B, Kyu T, Jákli A. Flexo-Ionic Effect of Ionic Liquid Crystal Elastomers. Molecules. 2021; 26(14):4234. https://doi.org/10.3390/molecules26144234
Chicago/Turabian StyleRajapaksha, C. P. Hemantha, M. D. Tharindupriya Gunathilaka, Suresh Narute, Hamad Albehaijan, Camilo Piedrahita, Pushpa Paudel, Chenrun Feng, Björn Lüssem, Thein Kyu, and Antal Jákli. 2021. "Flexo-Ionic Effect of Ionic Liquid Crystal Elastomers" Molecules 26, no. 14: 4234. https://doi.org/10.3390/molecules26144234
APA StyleRajapaksha, C. P. H., Gunathilaka, M. D. T., Narute, S., Albehaijan, H., Piedrahita, C., Paudel, P., Feng, C., Lüssem, B., Kyu, T., & Jákli, A. (2021). Flexo-Ionic Effect of Ionic Liquid Crystal Elastomers. Molecules, 26(14), 4234. https://doi.org/10.3390/molecules26144234