Secondary Metabolites from Marine Sources with Potential Use as Leads for Anticancer Applications
Abstract
:1. Introduction
2. Secondary Metabolites from Marine Organisms with Cytotoxic Activity
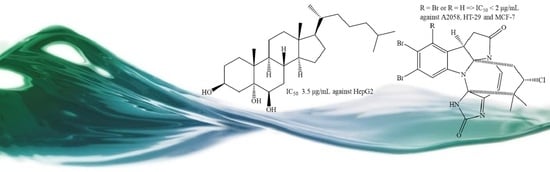
2.1. Securamines
2.2. Terpenoids
| Compound | Source a | Cell Lines | IC50 (μM) | [Ref.] b |
|---|---|---|---|---|
| Cholest-5-ene-3β-ol (6) | D. setosum D. savignyi | HeLa | >258 c | [35,56,57] |
| 5α,8α-Epidioxycholest-6-en-3β-ol (7) | D. setosum D. savignyi | HeLa | 29.04 ± 6.58 c | [35,56,57] |
| 5α,8α-Epidioxycholest-6,9(11)-en-3β-ol (8) | D. setosum | HeLa | 52.58 ± 15.24 c | [35] |
| Cholest-5-ene-3β-ol-sulphate (9) | D. setosum D. savignyi | HeLa | >258 c | [35,57] |
| Heterofuscesterol A (10) | H. fuscescens | MCF-7 | 72.57 ± 12.09 c | [37] |
| OVK-18 | 94.80 ± 7.94 c | |||
| Heterofuscesterol B (11) | H. fuscescens | MCF-7 | >262.79 c | [37] |
| OVK-18 | >262.79 c | |||
| 3β,5α,6β-Trihydroxyandrosta-17-one (12) | H. fuscescens | MCF-7 | >310.14 c | [37] |
| OVK-18 | >310.14 c | |||
| Gorgost-3β,5α,6β,11α-tetrol (13) | H. fuscescens H. crassiloba Sarcophyton sp. H. ghardaqensis | MCF-7 | >100 | [32,59,60,61,64] |
| 11α-Acetoxy-gorgost-3β,5α,6β-triol (14) | H. fuscescens Sarcophyton sp. H. ghardaqensis | MCF-7 | 33.2 | [32,60,61] |
| 3β-Acetoxy-gorgost-5α,6β,11α-triol (15) | H. fuscescens | MCF-7 | >100 | [32] |
| (R)-23-Methylergosta-20-ene-3β,5α,6β,17α-tetrol (16) | H. fuscescens | MCF-7 | 25.1 | [32] |
| Gorgost-5(E)-ene-3β-ol (17) | H. fuscescens H. ghardaqensis L. lobophytum | MCF-7 | >100 | [32,61,62] |
| 5α,6α-Epoxyergost-7-en-3β-ol (18) | M. vulgaris | HCT-16 | >241.16 c | [36] |
| (E)-24-Methylenecholestan-22-ene-3β,5α,6β-triol (19) | Sinularia sp. | HepG2 | 37.30 | [58] |
| HeLa | 19.32 | |||
| 24-Methylenecholesta-3β,5α,6β-triol (20) | Sinularia sp. C. copiosa | HepG2 | 13.36 | [58,65] |
| HeLa | 16.55 | |||
| (E)-24-Methylcholest-22-ene-3β,5α,6β-triol (21) | Sinularia sp. C. copiosa | HepG2 | 13.66 | [58,65] |
| HeLa | 18.31 | |||
| 24-Methylcholesta-3β,5α,6β-triol (22) | Sinularia sp. C. copiosa | HepG2 | 12.40 | [58,65] |
| HeLa | 8.82 | |||
| Cholest-3β,5α,6β-triol (23) | Sinularia sp. C. copiosa | HepG2 | 8.36 | [58,65] |
| HeLa | 16.48 | |||
| (10S,11S)-Epoxyeleganediol (24) | B. bifurcata | MDA-MB-231 | >310.09 c | [40] |
| (14R)-14,15-Epoxyeleganediol (25) | B. bifurcata | MDA-MB-231 | With 310.09 μM inhibited 78.8% c | [40] |
| (11R)-11-Hydroxyeleganediol (26) | B. bifurcata | MDA-MB-231 | >310.09 c | [40] |
| (11S)-11-Hydroxyeleganediol (27) | B. bifurcata | MDA-MB-231 | >312 c | [40] |
| Eleganolone (28) | B. bifurcata | MDA-MB-231 | 42.70 c | [40] |
| Dehydroderivative (29) | B. bifurcata | MDA-MB-231 | 109.30 c | [40] |
| 20-Hydroxygeranylgeraniol (30) | B. bifurcata | MDA-MB-231 | 32.63 c | [40] |
| 16-Hydroxygeranylgeraniol (31) | B. bifurcata | MDA-MB-231 | 47.31 c | [40] |
| Heterofusceterpene A (32) | H. fuscescens | MCF-7 | 86.45 ± 12.00 c | [37] |
| OVK-18 | 141.64 ± 9.80 c | |||
| (6R,11R)-(−)-Furodysinin (33) | H. infucata | HeLa | >474.76 c | [38] |
| Loliolide (34) | B. bifurcata | MDA-MB-231 | >509.57 c | [40] |
| Fucoxanthin analogue (35) | B. bifurcata | MDA-MB-231 | >326.38 c | [40] |
| Fucoxanthin (36) | B. bifurcata | MDA-MB-231 | >151.77 c | [40] |
| (1R,2S,4R,5R)-2-Bromo-4,5-dichloro-1-[(E)-2-chlorovinyl]-1,5-dimethylcyclohexane (37) | P. capillaces | HT-29 | 176.30 ± 27.08 c | [41] |
| LS174 | 155.30 ± 14.07 c |
2.3. Other Secondary Metabolites
3. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Cancer Today. Available online: https://gco.iarc.fr/today/home (accessed on 23 May 2021).
- World Health Organization. Cancer Tomorrow. Available online: https://gco.iarc.fr/tomorrow/en (accessed on 24 May 2021).
- White, M.C.; Holman, D.M.; Boehm, J.E.; Peipins, L.A.; Grossman, M.; Jane Henley, S. Age and Cancer Risk. Am. J. Prev. Med. 2014, 46, S7–S15. [Google Scholar] [CrossRef] [Green Version]
- Gurib-Fakim, A. Medicinal plants: Traditions of yesterday and drugs of tomorrow. Mol. Asp. Med. 2006, 27, 1–93. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- World Health Organization. Global Status Report on Noncommunicable Diseases 2014; World Health Organization: Geneva, Switzerland, 2014. [Google Scholar]
- Mullard, A. FDA approves first immunotherapy combo. Nat. Rev. Drug Discov. 2015, 14, 739. [Google Scholar] [CrossRef]
- De Lartigue, J. Tumor heterogeneity: A central foe in the war on cancer. J. Community Support. Oncol. 2016, 16, e167–e174. [Google Scholar] [CrossRef]
- Nikolaou, M.; Pavlopoulou, A.; Georgakilas, A.G.; Kyrodimos, E. The challenge of drug resistance in cancer treatment: A current overview. Clin. Exp. Metastasis 2018, 35, 309–318. [Google Scholar] [CrossRef] [PubMed]
- Sawadogo, W.; Boly, R.; Cerella, C.; Teiten, M.; Dicato, M.; Diederich, M. A Survey of Marine Natural Compounds and Their Derivatives with Anti-Cancer Activity Reported in 2012. Molecules 2015, 20, 7097–7142. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, A.P.; Ohlsson, R.; Henikoff, S. The epigenetic progenitor origin of human cancer. Nat. Rev. Genet. 2006, 7, 21–33. [Google Scholar] [CrossRef]
- Sun, W. Recent advances in cancer immunotherapy. J. Hematol. Oncol. 2017, 10, 96. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Torres, V.; Encinar, J.; Herranz-López, M.; Pérez-Sánchez, A.; Galiano, V.; Barrajón-Catalán, E.; Micol, V. An Updated Review on Marine Anticancer Compounds: The Use of Virtual Screening for the Discovery of Small-Molecule Cancer Drugs. Molecules 2017, 22, 1037. [Google Scholar] [CrossRef]
- Seca, A.M.L.; Pinto, D.C.G.A. Plant Secondary Metabolites as Anticancer Agents: Successes in Clinical Trials and Therapeutic Application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef] [Green Version]
- Newman, D.J.; Cragg, G.M. Natural Products As Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [Green Version]
- Walsh, V.; Goodman, J. Cancer chemotherapy, biodiversity, public and private property: The case of the anti-cancer drug Taxol. Soc. Sci. Med. 1999, 49, 1215–1225. [Google Scholar] [CrossRef]
- DrugBank Online. Database for Drug and Drug Target Info. Available online: https://go.drugbank.com/ (accessed on 24 May 2021).
- Dyshlovoy, S.; Honecker, F. Marine Compounds and Cancer: 2017 Updates. Mar. Drugs 2018, 16, 41. [Google Scholar] [CrossRef] [Green Version]
- Boeuf, G. Marine biodiversity characteristics. C. R. Biol. 2011, 334, 435–440. [Google Scholar] [CrossRef]
- Khalifa, S.A.M.; Elias, N.; Farag, M.A.; Chen, L.; Saeed, A.; Hegazy, M.-E.F.; Moustafa, M.S.; Abd El-Wahed, A.; Al-Mousawi, S.M.; Musharraf, S.G.; et al. Marine Natural Products: A Source of Novel Anticancer Drugs. Mar. Drugs 2019, 17, 491. [Google Scholar] [CrossRef] [Green Version]
- Sithranga Boopathy, N.; Kathiresan, K. Anticancer Drugs from Marine Flora: An Overview. J. Oncol. 2010, 2010, 214186. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, D.J. Highlights of marine natural products chemistry (1972–1999). Nat. Prod. Rep. 2000, 17, 1–6. [Google Scholar] [CrossRef]
- Shinde, P.; Banerjee, P.; Mandhare, A. Marine natural products as source of new drugs: A patent review (2015–2018). Expert Opin. Ther. Pat. 2019, 29, 283–309. [Google Scholar] [CrossRef]
- Wali, A.F.; Majid, S.; Rasool, S.; Shehada, S.B.; Abdulkareem, S.K.; Firdous, A.; Beigh, S.; Shakeel, S.; Mushtaq, S.; Akbar, I.; et al. Natural products against cancer: Review on phytochemicals from marine sources in preventing cancer. Saudi Pharm. J. 2019, 27, 767–777. [Google Scholar] [CrossRef]
- Srinivasan, N.; Dhanalakshmi, S.; Pandian, P. Encouraging Leads from Marine Sources for Cancer Therapy A Review Approach. Pharmacogn. J. 2020, 12, 1475–1481. [Google Scholar] [CrossRef]
- Kamyab, E.; Rohde, S.; Kellermann, M.Y.; Schupp, P.J. Chemical Defense Mechanisms and Ecological Implications of Indo-Pacific Holothurians. Molecules 2020, 25, 4808. [Google Scholar] [CrossRef]
- Woo, S.-Y.; Win, N.N.; Wong, C.P.; Ito, T.; Hoshino, S.; Ngwe, H.; Aye, A.A.; Han, N.M.; Zhang, H.; Hayashi, F.; et al. Two new pyrrolo-2-aminoimidazoles from a Myanmarese marine sponge, Clathria prolifera. J. Nat. Med. 2018, 72, 803–807. [Google Scholar] [CrossRef] [PubMed]
- Shushizadeh, M.R.; Beigi Nasiri, M.; Ameri, A.-G.; Rajabzadeh Ghatrami, E.; Tavakoli, S. Preparation of the Persian Gulf Echinometra mathaei Organic Extracts and Investigation of Their Antibacterial Activity. Jundishapur J. Nat. Pharm. Prod. 2019, 14. [Google Scholar] [CrossRef]
- Korpayev, S.; Heydari, H.; Koc, A.; Gozcelioglu, B.; Konuklugil, B. Additional screening of bioactivities in the Turkish gorgonian Paramuricea clavata (Risso, 1826) with isolation of secondary metabolites. Cah. Biol. Mar. 2020, 61, 25–32. [Google Scholar] [CrossRef]
- Quiroz Lobo, Y.; Santafé Patiño, G.; Quirós-Rodríguez, J.A. Characterization of fatty acids and antimicrobial activity of the methanol extract of Holothuria princeps (Holothuriida: Holothuriidae). Rev. Biol. Trop. 2020, 69, 36–44. [Google Scholar] [CrossRef]
- Abdelkarem, F.M.; Desoky, E.-E.K.; Nafady, A.M.; Allam, A.E.; Mahdy, A.; Ashour, A.; Mohamed, G.A.; Miyamoto, T.; Shimizu, K. Two new polyhydroxylated steroids from Egyptian soft coral Heteroxenia fuscescens (Fam.; Xeniidae). Nat. Prod. Res. 2021, 35, 236–243. [Google Scholar] [CrossRef]
- Hansen, K.Ø.; Isaksson, J.; Bayer, A.; Johansen, J.A.; Andersen, J.H.; Hansen, E. Securamine Derivatives from the Arctic Bryozoan Securiflustra securifrons. J. Nat. Prod. 2017, 80, 3276–3283. [Google Scholar] [CrossRef]
- Hansen, K.Ø.; Hansen, I.K.Ø.; Richard, C.S.; Jenssen, M.; Andersen, J.H.; Hansen, E.H. Antimicrobial Activity of Securamines From the Bryozoan Securiflustra securifrons. Nat. Prod. Commun. 2021, 16. [Google Scholar] [CrossRef]
- Abdelkarem, F.M.; Desoky, E.-E.K.; Nafady, A.M.; Allam, A.E.; Mahdy, A.; Ashour, A.; Shimizu, K. Diadema setosum: Isolation of bioactive secondary metabolites with cytotoxic activity toward human cervical cancer. Nat. Prod. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- Konuklugil, B.; Sertdemir, M.; Heydari, H.; Koc, A. Isolation and bioactivities screening of turkish Microcosmus vulgaris. Turkish J. Fish. Aquat. Sci. 2019, 19, 653–659. [Google Scholar] [CrossRef]
- Abdelkarem, F.M.; Desoky, E.-E.K.; Nafady, A.M.; Allam, A.E.; Mahdy, A.; Nagata, M.; Miyamoto, T.; Shimizu, K. Isolation of new secondary metabolites from gorgonian soft coral Heteroxenia fuscescens collected from Red Sea. Phytochem. Lett. 2020, 36, 156–161. [Google Scholar] [CrossRef]
- Mudianta, I.W.; Martiningsih, N.W.; Prasetia, I.N.D.; Nursid, M. Bioactive Terpenoid from the Balinese Nudibranch Hypselodoris infucata. Indones. J. Pharm. 2016, 27, 104. [Google Scholar] [CrossRef]
- Pereira, R.B.; Pereira, D.M.; Jiménez, C.; Rodríguez, J.; Nieto, R.M.; Videira, R.A.; Silva, O.; Andrade, P.B.; Valentão, P. Anti-Inflammatory Effects of 5α,8α-Epidioxycholest-6-en-3β-ol, a Steroidal Endoperoxide Isolated from Aplysia depilans, Based on Bioguided Fractionation and NMR Analysis. Mar. Drugs 2019, 17, 330. [Google Scholar] [CrossRef] [Green Version]
- Smyrniotopoulos, V.; Firsova, D.; Fearnhead, H.; Grauso, L.; Mangoni, A.; Tasdemir, D. Density Functional Theory (DFT)-Aided Structure Elucidation of Linear Diterpenes from the Irish Brown Seaweed Bifurcaria bifurcata. Mar. Drugs 2021, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Tarhouni-Jabberi, S.; Zakraoui, O.; Ioannou, E.; Riahi-Chebbi, I.; Haoues, M.; Roussis, V.; Kharrat, R.; Essafi-Benkhadir, K. Mertensene, a Halogenated Monoterpene, Induces G2/M Cell Cycle Arrest and Caspase Dependent Apoptosis of Human Colon Adenocarcinoma HT29 Cell Line through the Modulation of ERK-1/-2, AKT and NF-κB Signaling. Mar. Drugs 2017, 15, 221. [Google Scholar] [CrossRef] [Green Version]
- Zubia, M.; Thomas, O.P.; Soulet, S.; Demoy-Schneider, M.; Saulnier, D.; Connan, S.; Murphy, E.C.; Tintillier, F.; Stiger-Pouvreau, V.; Petek, S. Potential of tropical macroalgae from French Polynesia for biotechnological applications. J. Appl. Phycol. 2020, 32, 2343–2362. [Google Scholar] [CrossRef]
- Putra, M.Y.; Wibowo, J.T.; Murniasih, T.; Rasyid, A. Evaluation of antibacterial activity from Indonesian marine soft coral Sinularia sp. AIP Conf. Proc. 2016, 1744, 020039. [Google Scholar] [CrossRef] [Green Version]
- Ciftci, H.I.; Can, M.; Ellakwa, D.E.; Suner, S.C.; Ibrahim, M.A.; Oral, A.; Sekeroglu, N.; Özalp, B.; Otsuka, M.; Fujita, M.; et al. Anticancer activity of Turkish marine extracts: A purple sponge extract induces apoptosis with multitarget kinase inhibition activity. Investig. New Drugs 2020, 38, 1326–1333. [Google Scholar] [CrossRef]
- Martins, R.M.; Nedel, F.; Guimarães, V.B.S.; da Silva, A.F.; Colepicolo, P.; de Pereira, C.M.P.; Lund, R.G. Macroalgae Extracts From Antarctica Have Antimicrobial and Anticancer Potential. Front. Microbiol. 2018, 9, 412. [Google Scholar] [CrossRef]
- De La Fuente, G.; Fontana, M.; Asnaghi, V.; Chiantore, M.; Mirata, S.; Salis, A.; Damonte, G.; Scarfì, S. The Remarkable Antioxidant and Anti-Inflammatory Potential of the Extracts of the Brown Alga Cystoseira amentacea var. stricta. Mar. Drugs 2020, 19, 2. [Google Scholar] [CrossRef]
- Abu-Khudir, R.; Ismail, G.A.; Diab, T. Antimicrobial, Antioxidant, and Anti-Tumor Activities of Sargassum linearifolium and Cystoseira crinita from Egyptian Mediterranean Coast. Nutr. Cancer 2021, 73, 829–844. [Google Scholar] [CrossRef]
- Ercolano, G.; De Cicco, P.; Ianaro, A. New drugs from the sea: Pro-apoptotic activity of sponges and algae derived compounds. Mar. Drugs 2019, 17, 31. [Google Scholar] [CrossRef] [Green Version]
- Korakas, P.; Chaffee, S.; Shotwell, J.B.; Duque, P.; Wood, J.L. Efficient construction of the securine A carbon skeleton. Proc. Natl. Acad. Sci. USA 2004, 101, 12054–12057. [Google Scholar] [CrossRef] [Green Version]
- Rahbaek, L.; Anthoni, U.; Christophersen, C.; Nielsen, P.H.; Petersen, B.O. Marine Alkaloids. 18. Securamines and Securines, HalogenatedIndole-Imidazole Alkaloids from the Marine Bryozoan Securiflustra securifrons. J. Org. Chem. 1996, 61, 887–889. [Google Scholar] [CrossRef]
- Rahbaek, L.; Christophersen, C. Marine Alkaloids. 19. Three New Alkaloids, Securamines E-G, from the Marine Bryozoan Securiflustra securifrons. J. Nat. Prod. 1997, 60, 175–177. [Google Scholar] [CrossRef]
- Peña-Morán, O.A.; Villarreal, M.L.; Álvarez-Berber, L.; Meneses-Acosta, A.; Rodríguez-López, V. Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from Bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules 2016, 21, 1013. [Google Scholar] [CrossRef]
- Rabi, T.; Bishayee, A. Terpenoids and breast cancer chemoprevention. Breast Cancer Res. Treat. 2009, 115, 223–239. [Google Scholar] [CrossRef]
- Liby, K.T.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Rev. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.H.A.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites in vitro and in vivo anticancer activity. Mar. Drugs 2018, 16, 410. [Google Scholar] [CrossRef] [Green Version]
- Van Minn, C.; Van Kiem, P.; Huong, L.M.; Kim, Y.H. Cytotoxic constituents of Diadema setosum. Arch. Pharmacal Res. 2004, 27, 734–737. [Google Scholar] [CrossRef]
- Thao, N.P.; Bach, D.N.; Van Thanh, N.; Giang, P.M.; Nam, N.H.; Cuong, N.X.; Kim, Y.H.; Van Kiem, P.; Van Minh, C. Sterols isolated from the sea urchin Diadema savignyi. Vietnam. J. Chem. 2015, 53, 37–41. [Google Scholar] [CrossRef]
- Sun, H.; Liu, F.; Feng, M.-R.; Peng, Q.; Liao, X.-J.; Liu, T.-T.; Zhang, J.; Xu, S.-H. Isolation of a new cytotoxic polyhydroxysterol from the South China Sea soft coral Sinularia sp. Nat. Prod. Res. 2016, 30, 2819–2824. [Google Scholar] [CrossRef] [PubMed]
- Umeyama, A.; Shoji, N.; Ozeki, M.; Arihara, S. Sarcoaldesterols A and B, Two New Polyhydroxylated Sterols from the Soft Coral Sarcophyton sp. J. Nat. Prod. 1996, 59, 894–895. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, H.; Wang, P.; Gong, W.; Xue, M.; Zhang, H.; Liu, T.; Liu, B.; Yi, Y.; Zhang, W. Bioactive Polyoxygenated Steroids from the South China Sea Soft Coral, Sarcophyton sp. Mar. Drugs 2013, 11, 775–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elshamy, A.I.; Abdel-Razik, A.F.; Nassar, M.I.; Mohamed, T.K.; Ibrahim, M.A.; El-Kousy, S.M. A new gorgostane derivative from the Egyptian Red Sea soft coral Heteroxenia ghardaqensis. Nat. Prod. Res. 2013, 27, 1250–1254. [Google Scholar] [CrossRef]
- Hegazy, M.-E.F.; Mohamed, T.A.; Elshamy, A.I.; Hassanien, A.A.; Abdel-Azim, N.S.; Shreadah, M.A.; Abdelgawad, I.I.; Elkady, E.M.; Paré, P.W. A new steroid from the Red Sea soft coral Lobophytum lobophytum. Nat. Prod. Res. 2016, 30, 340–344. [Google Scholar] [CrossRef]
- Li, R.; Shao, C.-L.; Qi, X.; Li, X.-B.; Li, J.; Sun, L.-L.; Wang, C.-Y. Polyoxygenated Sterols from the South China Sea Soft Coral Sinularia sp. Mar. Drugs 2012, 10, 1422–1432. [Google Scholar] [CrossRef]
- Cheng, Z.-B.; Xiao, H.; Fan, C.-Q.; Lu, Y.-N.; Zhang, G.; Yin, S. Bioactive polyhydroxylated sterols from the marine sponge Haliclona crassiloba. Steroids 2013, 78, 1353–1358. [Google Scholar] [CrossRef]
- Notaro, G.; Piccialli, V.; Sica, D.; Corriero, G. 3β,5α,6β-Trihydroxylated Sterols with a Saturated Nucleus from Two Populations of the Marine Sponge Cliona copiosa. J. Nat. Prod. 1991, 54, 1570–1575. [Google Scholar] [CrossRef]
- Andrade, L.C.R.; de Almeida, M.J.B.M.; Paixão, J.A.; Carvalho, J.F.S.; Melo, M.L.S. e 3beta,5alpha,6beta-Trihydroxyandrostan-17-one. Available online: http://scripts.iucr.org/cgi-bin/paper?S1600536811011706 (accessed on 25 May 2021).
- Indrayanto, G.; Putra, G.S.; Suhud, F. Chapter six—Validation of in-vitro bioassay methods: Application in herbal drug research. In Profiles of Drug Substances, Excipients and Related Methodology; Al-Majed, A.A., Ed.; Academic Press: Cambridge, MA, USA, 2021; Volume 46, pp. 273–307. [Google Scholar] [CrossRef]
- Roje-Busatto, R.; Ujević, I. PSP toxins profile in ascidian Microcosmus vulgaris (Heller, 1877) after human poisoning in Croatia (Adriatic Sea). Toxicon 2014, 79, 28–36. [Google Scholar] [CrossRef]
- Verseveldt, J. A revision of the genus Sinularia May (Octocorallia, Alcyonacea). Zool. Verh. 1980, 179, 1–128. Available online: https://repository.naturalis.nl/pub/317650 (accessed on 25 May 2021).
- Van Ofwegen, L.P.; Benayahu, Y.; McFadden, C.S. Sinularia leptoclados (Ehrenberg, 1834) (Cnidaria, Octocorallia) re-examined. Zookeys 2013, 272, 29–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiry, M.D.; Guiry, G. Algaebase: Listing the World’s Algae. Available online: https://www.algaebase.org/ (accessed on 25 May 2021).
- Muñoz, J.; Culioli, G.; Köck, M. Linear diterpenes from the marine brown alga Bifurcaria bifurcata: A chemical perspective. Phytochem. Rev. 2013, 12, 407–424. [Google Scholar] [CrossRef]
- Rosa, G.P.; Tavares, W.R.; Sousa, P.M.; Pagès, A.K.; Seca, A.M.L.; Pinto, D.C.G.A. Seaweed secondary metabolites with beneficial health effects: An overview of successes in in-vivo studies and clinical trials. Mar. Drugs 2020, 18, 8. [Google Scholar] [CrossRef] [Green Version]
- Weber, J.F.; Fuhrman, F.A.; Fuhrman, G.J.; Mosher, H.S. Isolation of allantoin and adenosine from the marine sponge Tethya aurantia. Comp. Biochem. Physiol. Part B Comp. Biochem. 1981, 70, 799–801. [Google Scholar] [CrossRef]
- Abou-hussein, D.R.; Badr, J.M.; Youssef, D.T.A. Nucleoside constituents of the Egyptian tunicate Eudistoma laysani. Nat. Prod. Sci. 2007, 13, 229–233. [Google Scholar]
- Shin, J.; Seo, Y. Isolation of New Ceramides from the Gorgonian Acabaria undulata. J. Nat. Prod. 1995, 58, 948–953. [Google Scholar] [CrossRef]
- Rahelivao, M.P.; Lübken, T.; Gruner, M.; Kataeva, O.; Ralambondrahety, R.; Andriamanantoanina, H.; Checinski, M.P.; Bauer, I.; Knölker, H.-J. Isolation and structure elucidation of natural products of three soft corals and a sponge from the coast of Madagascar. Org. Biomol. Chem. 2017, 15, 2593–2608. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Cell Lines Tested | IC50 b (μM) | ||||
|---|---|---|---|---|---|
| Securamine H (1) | Securamine I (2) | Securamine J (3) | Securamine C (4) | Securamine E (5) | |
| A2058 | 1.4 | 2.7 | >50 | 20 | 6.7 |
| HT-29 | 1.9 | 2.5 | 21 | 10 | |
| MCF-7 | 2.1 | 2.4 | 23 | 8.5 | |
| MRC-5 | 2.7 | 5.3 | 30 | 9.6 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veríssimo, A.C.S.; Pacheco, M.; Silva, A.M.S.; Pinto, D.C.G.A. Secondary Metabolites from Marine Sources with Potential Use as Leads for Anticancer Applications. Molecules 2021, 26, 4292. https://doi.org/10.3390/molecules26144292
Veríssimo ACS, Pacheco M, Silva AMS, Pinto DCGA. Secondary Metabolites from Marine Sources with Potential Use as Leads for Anticancer Applications. Molecules. 2021; 26(14):4292. https://doi.org/10.3390/molecules26144292
Chicago/Turabian StyleVeríssimo, Ana C. S., Mário Pacheco, Artur M. S. Silva, and Diana C. G. A. Pinto. 2021. "Secondary Metabolites from Marine Sources with Potential Use as Leads for Anticancer Applications" Molecules 26, no. 14: 4292. https://doi.org/10.3390/molecules26144292
APA StyleVeríssimo, A. C. S., Pacheco, M., Silva, A. M. S., & Pinto, D. C. G. A. (2021). Secondary Metabolites from Marine Sources with Potential Use as Leads for Anticancer Applications. Molecules, 26(14), 4292. https://doi.org/10.3390/molecules26144292