Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract
Abstract
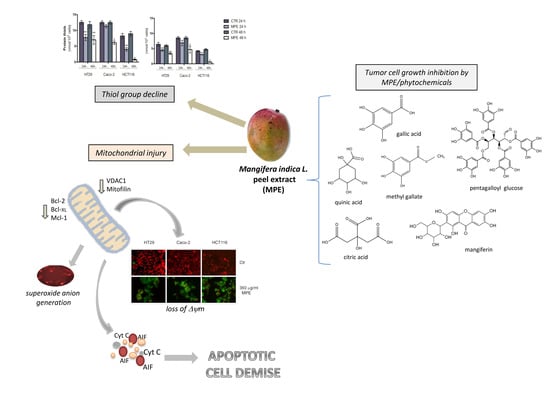
:1. Introduction
2. Results
2.1. MPE Treatment Promotes a Dramatic Decrease in Thiol Group Content, Changes in MnSOD Enzyme, and Mitochondrial Superoxide Anion Generation
2.2. MPE Causes Mitochondrial Membrane Potential Dissipation in Colon Cancer cells
2.3. MPE Treatment Affects the Level of Some Antiapoptotic Members of the Bcl-2 Family
2.4. MPE-Mediated Mitochondrial Injury Promotes the Mitochondrial Release of the Apoptogenic Proteins Cytochrome C and AIF
2.5. Analysis of the Cytotoxic Effects Exerted by Phytochemicals Contained in MPE
3. Discussion
4. Materials and Methods
4.1. Cell Cultures and Chemicals
4.2. Preparation of Mangifera Indica Peel Extracts
4.3. Analysis of Cell Viability by MTT Assay
4.4. Analysis of Thiol Group Content
4.5. Detection of Mitochondrial Superoxide Anion Production
4.6. Analysis of Mitochondrial Membrane Potential
4.7. Protein Extraction and Western Blot Analysis
4.8. Preparation of Mitochondrial and Cytosolic Fractions
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kong, H.; Chandel, N.S. Regulation of Redox Balance in Cancer and T Cells. J. Biol. Chem. 2018, 293, 7499–7507. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS Signalling in the Biology of Cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Chuang, C.-C.; Wu, S.; Zuo, L. Reactive Oxygen Species in Redox Cancer Therapy. Cancer Lett. 2015, 367, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Bienertova-Vasku, J.; Lenart, P.; Scheringer, M. Eustress and Distress: Neither Good Nor Bad, but Rather the Same? BioEssays 2020, 42, 1900238. [Google Scholar] [CrossRef]
- Farhood, B.; Najafi, M.; Salehi, E.; Hashemi Goradel, N.; Nashtaei, M.S.; Khanlarkhani, N.; Mortezaee, K. Disruption of the Redox Balance with Either Oxidative or Anti-oxidative Overloading as a Promising Target for Cancer Therapy. J. Cell. Biochem. 2019, 120, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shadel, G.S.; Horvath, T.L. Mitochondrial ROS Signaling in Organismal Homeostasis. Cell 2015, 163, 560–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazat, J.-P.; Devin, A.; Ransac, S. Modelling Mitochondrial ROS Production by the Respiratory Chain. Cell. Mol. Life Sci. 2020, 77, 455–465. [Google Scholar] [CrossRef]
- Emanuele, S.; D’Anneo, A.; Calvaruso, G.; Cernigliaro, C.; Giuliano, M.; Lauricella, M. The Double-Edged Sword Profile of Redox Signaling: Oxidative Events As Molecular Switches in the Balance between Cell Physiology and Cancer. Chem. Res. Toxicol. 2018, 31, 201–210. [Google Scholar] [CrossRef]
- Novo, N.; Ferreira, P.; Medina, M. The Apoptosis-inducing Factor Family: Moonlighting Proteins in the Crosstalk between Mitochondria and Nuclei. IUBMB Life 2021, 73, 568–581. [Google Scholar] [CrossRef]
- Kowalska, M.; Piekut, T.; Prendecki, M.; Sodel, A.; Kozubski, W.; Dorszewska, J. Mitochondrial and Nuclear DNA Oxidative Damage in Physiological and Pathological Aging. DNA Cell Biol. 2020, 39, 1410–1420. [Google Scholar] [CrossRef]
- Jahurul, M.H.A.; Zaidul, I.S.M.; Ghafoor, K.; Al-Juhaimi, F.Y.; Nyam, K.-L.; Norulaini, N.A.N.; Sahena, F.; Mohd Omar, A.K. Mango (Mangifera Indica L.) by-Products and Their Valuable Components: A Review. Food Chem. 2015, 183, 173–180. [Google Scholar] [CrossRef]
- Lauricella, M.; Emanuele, S.; Calvaruso, G.; Giuliano, M.; D’Anneo, A. Multifaceted Health Benefits of Mangifera Indica L. (Mango): The Inestimable Value of Orchards Recently Planted in Sicilian Rural Areas. Nutrients 2017, 9, 525. [Google Scholar] [CrossRef]
- Gentile, C.; Di Gregorio, E.; Di Stefano, V.; Mannino, G.; Perrone, A.; Avellone, G.; Sortino, G.; Inglese, P.; Farina, V. Food Quality and Nutraceutical Value of Nine Cultivars of Mango (Mangifera Indica L.) Fruits Grown in Mediterranean Subtropical Environment. Food Chem. 2019, 277, 471–479. [Google Scholar] [CrossRef]
- Testa, R.; Tudisca, S.; Schifani, G.; Di Trapani, A.; Migliore, G. Tropical Fruits as an Opportunity for Sustainable Development in Rural Areas: The Case of Mango in Small-Sized Sicilian Farms. Sustainability 2018, 10, 1436. [Google Scholar] [CrossRef] [Green Version]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical Composition of Mango (Mangifera Indica L.) Fruit: Nutritional and Phytochemical Compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Lebaka, V.R.; Wee, Y.-J.; Ye, W.; Korivi, M. Nutritional Composition and Bioactive Compounds in Three Different Parts of Mango Fruit. Int. J. Environ. Res. Public Health 2021, 18, 741. [Google Scholar] [CrossRef] [PubMed]
- Ediriweera, M.K.; Tennekoon, K.H.; Samarakoon, S.R. A Review on Ethnopharmacological Applications, Pharmacological Activities, and Bioactive Compounds of Mangifera Indica (Mango). Evid. Based Complement. Alternat. Med. 2017, 2017, 1–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, A.; Guo, X.; Fu, X.; Zhou, L.; Chen, Y.; Zhu, Y.; Yan, H.; Liu, R. Comparative Assessment of Phenolic Content and in Vitro Antioxidant Capacity in the Pulp and Peel of Mango Cultivars. Int. J. Mol. Sci. 2015, 16, 13507–13527. [Google Scholar] [CrossRef] [Green Version]
- Noratto, G.D.; Bertoldi, M.C.; Krenek, K.; Talcott, S.T.; Stringheta, P.C.; Mertens-Talcott, S.U. Anticarcinogenic Effects of Polyphenolics from Mango (Mangifera Indica) Varieties. J. Agric. Food Chem. 2010, 58, 4104–4112. [Google Scholar] [CrossRef]
- Banerjee, N.; Kim, H.; Krenek, K.; Talcott, S.T.; Mertens-Talcott, S.U. Mango Polyphenolics Suppressed Tumor Growth in Breast Cancer Xenografts in Mice: Role of the PI3K/AKT Pathway and Associated MicroRNAs. Nutr. Res. 2015, 35, 744–751. [Google Scholar] [CrossRef]
- Lauricella, M.; Lo Galbo, V.; Cernigliaro, C.; Maggio, A.; Palumbo Piccionello, A.; Calvaruso, G.; Carlisi, D.; Emanuele, S.; Giuliano, M.; D’Anneo, A. The Anti-Cancer Effect of Mangifera Indica L. Peel Extract Is Associated to ΓH2AX-Mediated Apoptosis in Colon Cancer Cells. Antioxidants 2019, 8, 422. [Google Scholar] [CrossRef] [Green Version]
- Kükürt, A.; Gelen, V.; Faruk Başer, Ö.; Ahmet Deveci, H.; Karapehlivan, M. Thiols: Role in Oxidative Stress-Related Disorders. In Lipid Peroxidation; IntechOpen: London, UK, 2021; Available online: http://www.intechopen.com/online-first/thiols-role-in-oxidative-stress-related-disorders (accessed on 20 March 2021). [CrossRef]
- Camara, A.K.S.; Zhou, Y.; Wen, P.-C.; Tajkhorshid, E.; Kwok, W.-M. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 2017, 8, 460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Von der Malsburg, K.; Müller, J.M.; Bohnert, M.; Oeljeklaus, S.; Kwiatkowska, P.; Becker, T.; Loniewska-Lwowska, A.; Wiese, S.; Rao, S.; Milenkovic, D.; et al. Dual Role of Mitofilin in Mitochondrial Membrane Organization and Protein Biogenesis. Dev. Cell 2011, 21, 694–707. [Google Scholar] [CrossRef] [Green Version]
- Borkan, S.C. The Role of BCL-2 Family Members in Acute Kidney Injury. Semin. Nephrol. 2016, 36, 237–250. [Google Scholar] [CrossRef]
- Gabellini, C.; Trisciuoglio, D.; Del Bufalo, D. Non-Canonical Roles of Bcl-2 and Bcl-XL Proteins: Relevance of BH4 Domain. Carcinogenesis 2017, 38, 579–587. [Google Scholar] [CrossRef]
- Tong, J.; Wang, P.; Tan, S.; Chen, D.; Nikolovska-Coleska, Z.; Zou, F.; Yu, J.; Zhang, L. Mcl-1 Degradation Is Required for Targeted Therapeutics to Eradicate Colon Cancer Cells. Cancer Res. 2017, 77, 2512–2521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uren, R.T.; Dewson, G.; Bonzon, C.; Lithgow, T.; Newmeyer, D.D.; Kluck, R.M. Mitochondrial Release of Pro-Apoptotic Proteins. J. Biol. Chem. 2005, 280, 2266–2274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasler, C.M. Functional Foods: Benefits, Concerns and Challenges—A Position Paper from the American Council on Science and Health. J. Nutr. 2002, 132, 3772–3781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Battino, M.; Forbes-Hernández, T.Y.; Gasparrini, M.; Afrin, S.; Cianciosi, D.; Zhang, J.; Manna, P.P.; Reboredo-Rodríguez, P.; Varela Lopez, A.; Quiles, J.L.; et al. Relevance of Functional Foods in the Mediterranean Diet: The Role of Olive Oil, Berries and Honey in the Prevention of Cancer and Cardiovascular Diseases. Crit. Rev. Food Sci. Nutr. 2019, 59, 893–920. [Google Scholar] [CrossRef] [PubMed]
- Gorzynik-Debicka, M.; Przychodzen, P.; Cappello, F.; Kuban-Jankowska, A.; Marino Gammazza, A.; Knap, N.; Wozniak, M.; Gorska-Ponikowska, M. Potential Health Benefits of Olive Oil and Plant Polyphenols. Int. J. Mol. Sci. 2018, 19, 686. [Google Scholar] [CrossRef] [Green Version]
- Arora, I.; Sharma, M.; Tollefsbol, T.O. Combinatorial Epigenetics Impact of Polyphenols and Phytochemicals in Cancer Prevention and Therapy. Int. J. Mol. Sci. 2019, 20, 4567. [Google Scholar] [CrossRef] [Green Version]
- Hazafa, A.; Rehman, K.-U.-; Jahan, N.; Jabeen, Z. The Role of Polyphenol (Flavonoids) Compounds in the Treatment of Cancer Cells. Nutr. Cancer 2020, 72, 386–397. [Google Scholar] [CrossRef] [PubMed]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Mirza, B.; Croley, C.R.; Ahmad, M.; Pumarol, J.; Das, N.; Sethi, G.; Bishayee, A. Mango (Mangifera Indica L.): A Magnificent Plant with Cancer Preventive and Anticancer Therapeutic Potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 2125–2151. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.M.; Dutra, T.S.; Simionatto, E.; Ré, N.; Kassuya, C.A.L.; Cardoso, C.A.L. Anti-Inflammatory Effects of Essential Oils from Mangifera Indica. Genet. Mol. Res. 2017, 16, 1. [Google Scholar] [CrossRef]
- Kim, H.; Venancio, V.P.; Fang, C.; Dupont, A.W.; Talcott, S.T.; Mertens-Talcott, S.U. Mango (Mangifera Indica L.) Polyphenols Reduce IL-8, GRO, and GM-SCF Plasma Levels and Increase Lactobacillus Species in a Pilot Study in Patients with Inflammatory Bowel Disease. Nutr. Res. 2020, 75, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Batool, N.; Ilyas, N.; Shabir, S.; Saeed, M.; Mazhar, R. A Mini-Review of Therapeutic Potential of Mangifera Indica L. Pak. J. Pharm. Sci. 2018, 31, 1441–1448. [Google Scholar]
- Corrales-Bernal, A.; Amparo Urango, L.; Rojano, B.; Maldonado, M.E. In vitro and in vivo effects of mango pulp (Mangifera indica cv. Azucar) in colon carcinogenesis. Arch. Latinoam. Nutr. 2014, 64, 16–23. [Google Scholar]
- Timisina, B.; Nadumane, V.K. Mango Seeds: A Potential Source for the Isolation of Bioactive Compounds with Anticancer Activity. Int. J. Pharm. Pharm. Sci. 2015, 7, 89–95. [Google Scholar]
- Daugas, E.; Susin, S.A.; Zamzami, N.; Ferri, K.F.; Irinopoulou, T.; Larochette, N.; Prévost, M.C.; Leber, B.; Andrews, D.; Penninger, J.; et al. Mitochondrio-Nuclear Translocation of AIF in Apoptosis and Necrosis. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2000, 14, 729–739. [Google Scholar] [CrossRef] [Green Version]
- Madungwe, N.B.; Feng, Y.; Lie, M.; Tombo, N.; Liu, L.; Kaya, F.; Bopassa, J.C. Mitochondrial Inner Membrane Protein (Mitofilin) Knockdown Induces Cell Death by Apoptosis via an AIF-PARP-Dependent Mechanism and Cell Cycle Arrest. Am. J. Physiol. Cell Physiol. 2018, 315, C28–C43. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Fábregas, J.; Díaz-Moreno, I.; González-Arzola, K.; Janocha, S.; Navarro, J.A.; Hervás, M.; Bernhardt, R.; Velázquez-Campoy, A.; Díaz-Quintana, A.; De la Rosa, M.A. Structural and Functional Analysis of Novel Human Cytochrome c Targets in Apoptosis. Mol. Cell. Proteom. 2014, 13, 1439–1456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Díaz-Moreno, I.; Velázquez-Cruz, A.; Curran-French, S.; Díaz-Quintana, A.; De la Rosa, M.A. Nuclear Cytochrome c—A Mitochondrial Visitor Regulating Damaged Chromatin Dynamics. FEBS Lett. 2018, 592, 172–178. [Google Scholar] [CrossRef] [Green Version]
- Yang, R.; Zhao, G.; Liang, S.; Zhang, Y.; Sun, L.; Chen, H.; Liu, D. Mitofilin Regulates Cytochrome c Release during Apoptosis by Controlling Mitochondrial Cristae Remodeling. Biochem. Biophys. Res. Commun. 2012, 428, 93–98. [Google Scholar] [CrossRef]
- Celesia, A.; Morana, O.; Fiore, T.; Pellerito, C.; D’Anneo, A.; Lauricella, M.; Carlisi, D.; De Blasio, A.; Calvaruso, G.; Giuliano, M.; et al. ROS-Dependent ER Stress and Autophagy Mediate the Anti-Tumor Effects of Tributyltin (IV) Ferulate in Colon Cancer Cells. Int. J. Mol. Sci. 2020, 21, 8135. [Google Scholar] [CrossRef] [PubMed]
- Moser, M.; Schneider, R.; Behnke, T.; Schneider, T.; Falkenhagen, J.; Resch-Genger, U. Ellman’s and Aldrithiol Assay as Versatile and Complementary Tools for the Quantification of Thiol Groups and Ligands on Nanomaterials. Anal. Chem. 2016, 88, 8624–8631. [Google Scholar] [CrossRef] [PubMed]
- D’Anneo, A.; Carlisi, D.; Lauricella, M.; Emanuele, S.; Di Fiore, R.; Vento, R.; Tesoriere, G. Parthenolide Induces Caspase-Independent and AIF-Mediated Cell Death in Human Osteosarcoma and Melanoma Cells. J. Cell. Physiol. 2013, 228, 952–967. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlisi, D.; D’Anneo, A.; Martinez, R.; Emanuele, S.; Buttitta, G.; Di Fiore, R.; Vento, R.; Tesoriere, G.; Lauricella, M. The Oxygen Radicals Involved in the Toxicity Induced by Parthenolide in MDA-MB-231 Cells. Oncol. Rep. 2014, 32, 167–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimauro, I.; Pearson, T.; Caporossi, D.; Jackson, M.J. A Simple Protocol for the Subcellular Fractionation of Skeletal Muscle Cells and Tissue. BMC Res. Notes 2012, 5, 513. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lo Galbo, V.; Lauricella, M.; Giuliano, M.; Emanuele, S.; Carlisi, D.; Calvaruso, G.; De Blasio, A.; Di Liberto, D.; D’Anneo, A. Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract. Molecules 2021, 26, 4328. https://doi.org/10.3390/molecules26144328
Lo Galbo V, Lauricella M, Giuliano M, Emanuele S, Carlisi D, Calvaruso G, De Blasio A, Di Liberto D, D’Anneo A. Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract. Molecules. 2021; 26(14):4328. https://doi.org/10.3390/molecules26144328
Chicago/Turabian StyleLo Galbo, Valentina, Marianna Lauricella, Michela Giuliano, Sonia Emanuele, Daniela Carlisi, Giuseppe Calvaruso, Anna De Blasio, Diana Di Liberto, and Antonella D’Anneo. 2021. "Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract" Molecules 26, no. 14: 4328. https://doi.org/10.3390/molecules26144328
APA StyleLo Galbo, V., Lauricella, M., Giuliano, M., Emanuele, S., Carlisi, D., Calvaruso, G., De Blasio, A., Di Liberto, D., & D’Anneo, A. (2021). Redox Imbalance and Mitochondrial Release of Apoptogenic Factors at the Forefront of the Antitumor Action of Mango Peel Extract. Molecules, 26(14), 4328. https://doi.org/10.3390/molecules26144328