Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity
Abstract
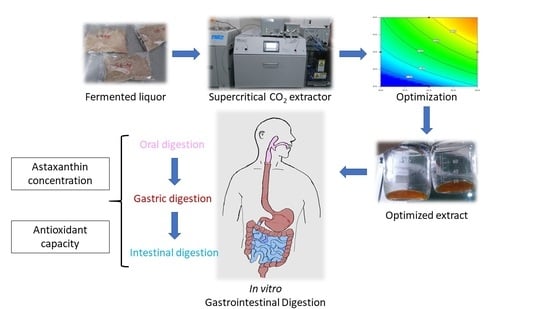
:1. Introduction
2. Results
2.1. Predictive Models
2.1.1. Extraction Yield
2.1.2. Antioxidant Capacity
2.1.3. Astaxanthin Concentration
2.2. Optimization
2.3. Antioxidant Characterization of Optimized Supercritical CO2 Extraction
2.4. Bioaccessibility of Astaxanthin of Optimized CO2 Extract
3. Discussion
4. Materials and Methods
4.1. Biological Material
4.2. Reagents and Chemicals
4.3. Supercritical CO2 Extraction
4.4. Extraction Yield
4.5. Radical-Scavenging Activity
4.5.1. ABTS
4.5.2. ORAC
4.6. Astaxanthin Content
4.7. Experimental Design
4.8. Simulated Gastrointestinal Digestion
4.9. Calculation of Bioaccessibility
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT. Fishery and Aquaculture Statistics Yearbook 2018; FAO: Rome, Italy, 2018. [Google Scholar]
- Mezzomo, N.; Martínez, J.; Maraschin, M.; Ferreira, S.R.S. Pink shrimp (P. brasiliensis and P. paulensis) residue: Supercritical fluid extraction of carotenoid fraction. J. Supercrit. Fluids 2013, 74, 22–33. [Google Scholar] [CrossRef]
- Montero, P.; Calvo, M.M.; Gómez-Guillén, M.C.; Gómez-Estaca, J. Microcapsules containing astaxanthin from shrimp waste as potential food coloring and functional ingredient: Characterization, stability, and bioaccessibility. LWT 2016, 70, 229–236. [Google Scholar] [CrossRef]
- Roy, V.C.; Getachew, A.T.; Cho, Y.-J.; Park, J.-S.; Chun, B.-S. Recovery and bio-potentialities of astaxanthin-rich oil from shrimp (Peneanus monodon) waste and mackerel (Scomberomous niphonius) skin using concurrent supercritical CO2 extraction. J. Supercrit. Fluids 2020, 159, 104773. [Google Scholar] [CrossRef]
- Saini, R.K.; Moon, S.H.; Keum, Y.-S. An updated review on use of tomato pomace and crustacean processing waste to recover commercially vital carotenoids. Food Res. Int. 2018, 108, 516–529. [Google Scholar] [CrossRef] [PubMed]
- Bahasan, S.H.O.; Satheesh, S.; Ba-akdah, M.A. Extraction of chitin from the shell wastes of two shrimp species Fenneropenaeus semisulcatus and Fenneropenaeus indicus using microorganisms. J. Aquat. Food Prod. Technol. 2017, 26, 390–405. [Google Scholar] [CrossRef]
- Ximenes, J.C.M.; Hissa, D.C.; Ribeiro, L.H.; Rocha, M.V.P.; Oliveira, E.G.; Melo, V.M.M. Sustainable recovery of protein-rich liquor from shrimp farming waste by lactic acid fermentation for application in tilapia feed. Braz. J. Microbiol. 2019, 50, 195–203. [Google Scholar] [CrossRef]
- Afonso, C.; Bandarra, N.M.; Nunes, L.; Cardoso, C. Tocopherols in seafood and aquaculture products. Crit. Rev. Food Sci. Nutr. 2016, 56, 128–140. [Google Scholar] [CrossRef]
- Higuera-Ciapara, I.; Felix-Valenzuela, L.; Goycoolea, F.M. Astaxanthin: A review of its chemistry and applications. Crit. Rev. Food Sci. Nutr. 2006, 46, 185–196. [Google Scholar] [CrossRef]
- Messina, C.M.; Manuguerra, S.; Renda, G.; Santulli, A. Biotechnological applications for the sustainable use of marine by-products: In vitro antioxidant and pro-apoptotic effects of astaxanthin extracted with supercritical CO2 from Parapeneus longirostris. Mar. Biotechnol. 2019, 21, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.X.; Xiong, F. Astaxanthin and its effects in inflammatory responses and inflammation-associated diseases: Recent advances and future directions. Molecules 2020, 25, 5342. [Google Scholar] [CrossRef] [PubMed]
- Prameela, K.; Venkatesh, K.; Immandi, S.B.; Kasturi, A.P.K.; Rama Krishna, C.; Murali Mohan, C. Next generation nutraceutical from shrimp waste: The convergence of applications with extraction methods. Food Chem. 2017, 237, 121–132. [Google Scholar] [CrossRef]
- Sowmya, P.R.-R.; Arathi, B.P.; Vijay, K.; Baskaran, V.; Lakshminarayana, R. Astaxanthin from shrimp efficiently modulates oxidative stress and allied cell death progression in MCF-7 cells treated synergistically with β-carotene and lutein from greens. Food Chem. Toxicol. 2017, 106, 58–69. [Google Scholar] [CrossRef]
- Stachowiak, B.; Szulc, P. Astaxanthin for the food industry. Molecules 2021, 26, 2666. [Google Scholar] [CrossRef] [PubMed]
- Ahmadkelayeh, S.; Hawboldt, K. Extraction of lipids and astaxanthin from crustacean by-products: A review on supercritical CO2 extraction. Trends Food Sci. Technol. 2020, 103, 94–108. [Google Scholar] [CrossRef]
- Brotosudarmo, T.H.P.; Limantara, L.; Setiyono, E. Structures of astaxanthin and their consequences for therapeutic application. Int. J. Food Sci. 2020, 2020, 2156582. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Gu, J.Y.; Luan, T.L.; Qiao, X.; Cao, Y.R.; Xue, C.H.; Xu, J. Influence of oil matrixes on stability, antioxidant activity, bioaccessibility and bioavailability of astaxanthin ester. J. Sci. Food Agric. 2021, 101, 1609–1617. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.R.; Palafox-Carlos, H.; Wall-Medrano, A.; Ayala-Zavala, J.F.; Chen, C.Y.O.; Robles-Sánchez, M.; Astiazaran-García, H.; Alvarez-Parrilla, E.; González-Aguilar, G.A. Phenolic compounds: Their journey after intake. Food Funct. 2014, 5, 189–197. [Google Scholar] [CrossRef]
- Bhaskar, N.; Suresh, P.V.; Sakhare, P.Z.; Sachindra, N.M. Shrimp biowaste fermentation with Pediococcus acidolactici CFR2182: Optimization of fermentation conditions by response surface methodology and effect of optimized conditions on deproteination/demineralization and carotenoid recovery. Enzyme Microb. Technol. 2007, 40, 1427–1434. [Google Scholar] [CrossRef]
- Sachindra, N.M.; Bhaskar, N.; Mahendrakar, N.S. Recovery of carotenoids from shrimp waste in organic solvents. J. Waste Manag. 2006, 26, 1092–1098. [Google Scholar] [CrossRef]
- De Holanda, H.D.; Netto, F.M. Recovery of components from shrimp (Xiphopenaeus kroyeri) processing waste by enzymatic hydrolysis. J. Food Sci. 2006, 71, C298–C303. [Google Scholar] [CrossRef]
- Mao, X.; Guo, N.; Sun, J.; Xue, C. Comprehensive utilization of shrimp waste based on biotechnological methods: A review. J. Clean. Prod. 2017, 143, 814–823. [Google Scholar] [CrossRef]
- Narayan, B.; Velappan, S.P.; Zituji, S.P.; Manjabhatta, S.N.; Gowda, L.R. Yield and chemical composition of fractions from fermented shrimp biowaste. Waste Manag. Res. 2010, 28, 64–70. [Google Scholar] [CrossRef]
- Molino, A.; Mehariya, S.; Di Sanzo, G.; Larocca, V.; Martino, M.; Leone, G.P.; Marino, T.; Chianese, S.; Balducchi, R.; Musmarra, D. Recent developments in supercritical fluid extraction of bioactive compounds from microalgae: Role of key parameters, technological achievements and challenges. J. CO2 Util. 2020, 36, 196–209. [Google Scholar] [CrossRef]
- Milán-Carrillo, J.; Montoya-Rodríguez, A.; Gutiérrez-Dorado, R.; Perales-Sánchez, X.; Reyes-Moreno, C. Optimization of extrusion process for producing high antioxidant instant amaranth (Amaranthus hypochondriacus L.) flour using response surface methodology. Appl. Math. 2012, 3, 1516–1525. [Google Scholar] [CrossRef] [Green Version]
- Zhu, C.-P.; Zhai, X.-C.; Li, L.-Q.; Wu, X.-X.; Li, B. Response surface optimization of ultrasound-assisted polysaccharides extraction from pomegranate peel. Food Chem. 2015, 177, 139–146. [Google Scholar] [CrossRef]
- Montgomery, D.C. Design and Analysis of Experiments, 5th ed.; Jhon Wiley: New York, NY, USA, 2002. [Google Scholar]
- Da Silva, R.P.F.F.; Rocha-Santos, T.A.P.; Duarte, A.C. Supercritical fluid extraction of bioactive compounds. Trends Anal. Chem. 2016, 76, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Radzali, S.A.; Masturah, M.; Baharin, B.S.; Rashidi, O.; Rahman, R.A. Optimisation of supercritical fluid extraction of astaxanthin from Penaeus monodon waste using ethanol-modified carbon dioxide. JESTEC 2016, 11, 722–736. [Google Scholar]
- Sánchez-Camargo, A.P.; Martinez-Correa, H.A.; Paviani, L.C.; Cabral, F.A. Supercritical CO2 extraction of lipids and astaxanthin from Brazilian redspotted shrimp waste (Farfantepenaeus paulensis). J. Supercrit. Fluids 2011, 56, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Gimeno, M.; Ramírez-Hernández, J.Y.; Mártinez-Ibarra, C.; Pacheco, N.; García-Arrazola, R.; Bárzana, E.; Shirai, K. One-solvent extraction of astaxanthin from lactic acid fermented shrimp wastes. J. Am. Chem. Soc. 2007, 55, 10345–10350. [Google Scholar] [CrossRef]
- Razi Parjikolaei, B.; Casas Cardoso, L.; Fernández Ponce, M.; Mantell, C.; Fretté, X.; Christensen, K. Northern Shrimp (Pandalus borealis) processing waste: Effect of supercritical fluid extraction technique on carotenoid extract concentration. Chem. Eng. Trans. 2015, 43, 1045–1050. [Google Scholar]
- Yang, X.; Zhang, Z.; Zheng, Q.; Zu, T.; Shu, Y. Optimization of supercritical CO2 extraction of astaxanthin from pacific white shrimp (Litopenaeus vannamei) using response surface methodology. TCSAE 2013, 29, 294–300. [Google Scholar]
- Radzali, S.A.; Baharin, B.S.; Othman, R.; Markom, M.; Rahman, R.A. Co-solvent selection for supercritical fluid extraction of astaxanthin and other carotenoids from Penaeus monodon waste. J. Oleo Sci. 2014, 63, 769–777. [Google Scholar] [CrossRef] [Green Version]
- Mohd Hatta, F.A.; Othman, R. 9—Carotenoids as potential biocolorants: A case study of astaxanthin recovered from shrimp waste. In Carotenoids: Properties, Processing and Applications; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2020; pp. 289–325. [Google Scholar]
- Chitchumroonchokchai, C.; Failla, M.L. Bioaccessibility and intestinal cell uptake of astaxanthin from salmon and commercial supplements. Food Res. Int. 2017, 99, 936–943. [Google Scholar] [CrossRef]
- Martinez-Alvarez, O.; Calvo, M.M.; Gomez-Estaca, J. Recent advances in astaxanthin micro/nanoencapsulation to improve its stability and functionality as a food ingredient. Mar. Drugs 2020, 18, 406. [Google Scholar] [CrossRef]
- Boonlao, N.; Shrestha, S.; Sadiq, M.B.; Anal, A.K. Influence of whey protein-xanthan gum stabilized emulsion on stability and in vitro digestibility of encapsulated astaxanthin. J. Food Eng. 2020, 272, 9. [Google Scholar] [CrossRef]
- Burgos-Diaz, C.; Opazo-Navarrete, M.; Soto-Anual, M.; Leal-Calderon, F.; Bustamante, M. Food-grade Pickering emulsion as a novel astaxanthin encapsulation system for making powder-based products: Evaluation of astaxanthin stability during processing, storage, and its bioaccessibility. Food Res. Int. 2020, 134, 9. [Google Scholar] [CrossRef]
- Ambati, R.R.; Phang, S.-M.; Ravi, S.; Aswathanarayana, R.G. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications—A review. Mar. Drugs 2014, 12, 128–152. [Google Scholar] [CrossRef] [PubMed]
- Maiani, G.; Periago Castón, M.J.; Catasta, G.; Toti, E.; Cambrodón, I.G.; Bysted, A.; Granado-Lorencio, F.; Olmedilla-Alonso, B.; Knuthsen, P.; Valoti, M.; et al. Carotenoids: Actual knowledge on food sources, intakes, stability and bioavailability and their protective role in humans. Mol. Nutr. Food Res. 2009, 53, S194–S218. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Grijalva, E.P.; Antunes-Ricardo, M.; Acosta-Estrada, B.A.; Gutiérrez-Uribe, J.A.; Basilio Heredia, J. Cellular antioxidant activity and in vitro inhibition of α-glucosidase, α-amylase and pancreatic lipase of oregano polyphenols under simulated gastrointestinal digestion. Food Res. Int. 2019, 116, 676–686. [Google Scholar] [CrossRef] [PubMed]
- Buniowska, M.; Carbonell-Capella, J.M.; Frigola, A.; Esteve, M.J. Bioaccessibility of bioactive compounds after non-thermal processing of an exotic fruit juice blend sweetened with Stevia rebaudiana. Food Chem. 2017, 221, 1834–1842. [Google Scholar] [CrossRef] [PubMed]
- De Souza Carvalho, L.M.; Lemos, M.C.M.; Sanches, E.A.; da Silva, L.S.; de Araújo Bezerra, J.; Aguiar, J.P.L.; das Chagas do Amaral Souza, F.; Alves Filho, E.G.; Campelo, P.H. Improvement of the bioaccessibility of bioactive compounds from Amazon fruits treated using high energy ultrasound. Ultrason. Sonochem. 2020, 67, 105148. [Google Scholar] [CrossRef]
- Yang, C.; Zhang, H.; Liu, R.; Zhu, H.; Zhang, L.; Tsao, R. Bioaccessibility, cellular uptake, and transport of astaxanthin isomers and their antioxidative effects in human intestinal epithelial caco-2 cells. J. Agric. Food Chem. 2017, 65, 10223–10232. [Google Scholar] [CrossRef]
- Su, F.; Huang, B.; Liu, J. The carotenoids of shrimps (Decapoda: Caridea and Dendrobranchiata) cultured in China. J. Crustacean Biol. 2018, 38, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Marcia, E.; Malespín, J.; Sánchez, M.; Benavente, M. Estudio de la fermentación láctica para la extracción de quitina a partir de desechos de crustáceos. Nexo Rev. Cient. 2011, 24, 33–42. [Google Scholar] [CrossRef] [Green Version]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of methods to determine antioxidant capacities. Food Anal. Methods 2009, 2, 41–60. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Hampsch-Woodill, M.; Flanagan, J.A.; Prior, R.L. High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format. J. Agric. Food Chem. 2002, 50, 4437–4444. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Lu, W.; Lv, M.; Wang, Y.; Ding, R.; Wang, L. Extraction and purification of astaxanthin from shrimp shells and the effects of different treatments on its content. Rev. Bras. Farmacogn. 2019, 29, 24–29. [Google Scholar] [CrossRef]
- Brodkorb, A.; Egger, L.; Alminger, M.; Alvito, P.; Assunção, R.; Ballance, S.; Bohn, T.; Bourlieu-Lacanal, C.; Boutrou, R.; Carrière, F.; et al. INFOGEST static in vitro simulation of gastrointestinal food digestion. Nat. Protoc. 2019, 14, 991–1014. [Google Scholar] [CrossRef]




| Regression Coefficients | Extraction Yield (%) | Antioxidant Capacity (ABTS) (mmol TE/g Lyophilized Liquor) | Astaxanthin Concentration (µg/g Lyophilized Liquor) | |||
|---|---|---|---|---|---|---|
| Coded | Uncoded | Coded | Uncoded | Coded | Uncoded | |
| Intercept | ||||||
| β0 | 9.49 | 36.99 | 0.89 | 4.41 | 0.40 | 0.5231 |
| Linear | ||||||
| β1 | 0.42 | 5.6398 × 10−3 | −0.033 | −0.011 | 0.073 | −1.1489 × 10−3 |
| β2 | −0.73 | −0.5583 | 0.21 | −0.026 | −8.903 × 10−3 | −8.9039 × 10−4 |
| β3 | −0.43 | −6.2817 | 0.15 | −1.11 | 0.090 | −0.0743 |
| Interaction | ||||||
| β12 | ||||||
| β13 | 0.40 | 2.66 × 10−3 | 0.080 | 5.3131 × 10−4 | ||
| β23 | 0.43 | 0.1213 | 0.24 | 0.011 | ||
| Statistical parameters | ||||||
| R2 | 0.9014 | 0.9014 | 0.9342 | 0.9342 | 0.9334 | 0.9334 |
| Regression (p value) | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 | 0.0001 |
| Lack of fit (p value) | 0.8241 | 0.8241 | 0.0686 | 0.0686 | 0.1073 | 0.1073 |
| Factor | Name | Optimum Level | Low Level | High Level | Coding |
|---|---|---|---|---|---|
| A | Pressure (bar) | 300 | 150 | 300 | Actual |
| B | Temperature (°C) | 60 | 40 | 60 | Actual |
| C | Flow rate (mL/min) | 6 | 2 | 6 | Actual |
| Response | Predicted Mean | Measured Data Mean | SE Prediction | 95% PI Low | 95% PI High |
| Extraction yield (%) | 11.17 | 12.62 | 0.75 | 9.5 | 12.86 |
| Antioxidant capacity (ABTS) (mmol TE/g) | 1.965 | 1.784 | 0.11 | 1.71 | 2.22 |
| Astaxanthin concentration (µg/g) | 0.6353 | 0.52 | 0.04 | 0.55 | 0.73 |
| Digestion Phase | Astaxanthin Concentration (µg/g Lyophilized Liquor) | Bioaccessibility of Astaxanthin (%) | ABTS (mmol TE/g Lyophilized Liquor) | Changes in ABTS Values during GD (%) | ORAC (mmol TE/g Lyophilized Liquor) | Changes in ORAC Values during GD (%) |
|---|---|---|---|---|---|---|
| Undigested | 0.52 ± 0.04 a | - | 1.78 ± 0.08 d | - | 5.44 ± 0.47 d | - |
| Oral | 0.48 ± 0.04 a | 92.30% a | 6.59 ± 0.32 b | 370.22% b | 21.14 ± 2.07 c | 388.6% c |
| Gastric | 0.19 ± 0.01 b | 36.53% b | 3.99 ± 0.28 c | 224.15% c | 59.09 ± 3.01 a | 1,086.21% a |
| Intestinal | ND | 13.73 ± 0.83 a | 771.34% a | 49.60 ± 2.09 b | 911.76% b |
| Sample Number | Pressure(bar) | Temperature (°C) | Flow Rate (mL/min) | Extraction Yield (%) | Antioxidant Capacity (ABTS) (mmol ET/g Lyophilized Liquor) | Astaxanthin Concentration (µg/g Lyophilized Liquor) |
|---|---|---|---|---|---|---|
| 1 | 150 | 40 | 4.00 | 9.309 | 0.8834 | 0.3374 |
| 2 | 300 | 40 | 4.00 | 10.015 | 0.5051 | 0.5024 |
| 3 | 150 | 60 | 4.00 | 8.317 | 0.9964 | 0.2787 |
| 4 | 300 | 60 | 4.00 | 8.510 | 1.0859 | 0.4482 |
| 5 | 150 | 50 | 2.00 | 9.841 | 1.145 | 0.3534 |
| 6 | 300 | 50 | 2.00 | 10.616 | 0.3565 | 0.3197 |
| 7 | 150 | 50 | 6.00 | 8.414 | 0.5996 | 0.3460 |
| 8 | 300 | 50 | 6.00 | 10.123 | 1.4093 | 0.6311 |
| 9 | 225 | 40 | 2.00 | 13.421 | 0.7061 | 0.2538 |
| 10 | 225 | 60 | 2.00 | 6.895 | 0.7132 | 0.3270 |
| 11 | 225 | 40 | 6.00 | 7.806 | 0.5743 | 0.5158 |
| 12 | 225 | 60 | 6.00 | 10.986 | 1.5247 | 0.4843 |
| 13 | 225 | 50 | 4.00 | 9.421 | 0.9421 | 0.3928 |
| 14 | 225 | 50 | 4.00 | 8.553 | 0.9366 | 0.4068 |
| 15 | 225 | 50 | 4.00 | 10.088 | 0.9934 | 0.4157 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cabanillas-Bojórquez, L.A.; Gutiérrez-Grijalva, E.P.; González-Aguilar, G.A.; López-Martinez, L.X.; Castillo-López, R.I.; Bastidas-Bastidas, P.d.J.; Heredia, J.B. Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules 2021, 26, 4465. https://doi.org/10.3390/molecules26154465
Cabanillas-Bojórquez LA, Gutiérrez-Grijalva EP, González-Aguilar GA, López-Martinez LX, Castillo-López RI, Bastidas-Bastidas PdJ, Heredia JB. Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules. 2021; 26(15):4465. https://doi.org/10.3390/molecules26154465
Chicago/Turabian StyleCabanillas-Bojórquez, Luis Angel, Erick Paul Gutiérrez-Grijalva, Gustavo Adolfo González-Aguilar, Leticia Xochitl López-Martinez, Ramón Ignacio Castillo-López, Pedro de Jesús Bastidas-Bastidas, and José Basilio Heredia. 2021. "Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity" Molecules 26, no. 15: 4465. https://doi.org/10.3390/molecules26154465
APA StyleCabanillas-Bojórquez, L. A., Gutiérrez-Grijalva, E. P., González-Aguilar, G. A., López-Martinez, L. X., Castillo-López, R. I., Bastidas-Bastidas, P. d. J., & Heredia, J. B. (2021). Valorization of Fermented Shrimp Waste with Supercritical CO2 Conditions: Extraction of Astaxanthin and Effect of Simulated Gastrointestinal Digestion on Its Antioxidant Capacity. Molecules, 26(15), 4465. https://doi.org/10.3390/molecules26154465