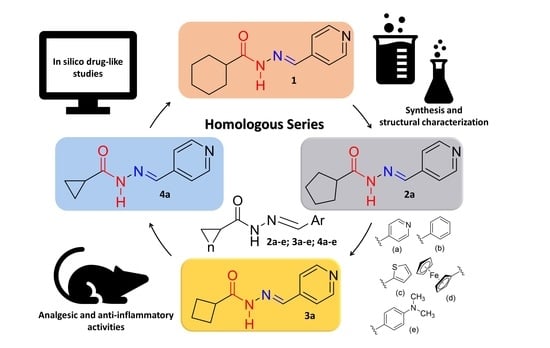
Design and Synthesis In Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Pharmacological Activities
3. Experimental Section
3.1. Chemistry
3.1.1. General Methods
3.1.2. General Procedure for Preparation of Cycloalkyl-hydrazides 8–10
Cyclopentanecarbohydrazide (8)
Cyclobutanecarbohydrazide (9)
Cyclopropanecarbohydrazide (10)
3.2. Antinociceptive and Anti-Inflammatory Pharmacological Evaluation
3.2.1. Animals
3.2.2. Reagents
3.2.3. Carrageenan-Induced Peritonitis
3.2.4. Acetic Acid-Induced Writhing Test
3.2.5. Formalin Induced Nociception
3.2.6. Carrageenan-Induced Nociception
3.2.7. Neuropathic Pain Model Induced by Spinal Nerve Ligation
Evaluation of the Thermal Hyperalgesia and Mechanical Allodynia Induced by SNL
Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, N.; Ranjana, R.; Kumari, M.; Kumar, B. A Review on Biological Activities of Hydrazone Derivatives. Intern. J. Pharmac. Clin. Res. 2016, 8, 162–166. [Google Scholar]
- Maia, R.C.; Tesch, R.; Fraga, C.A.M. Acylhydrazone derivatives: A patent review. Expert Opin. Ther. Pat. 2014, 24, 1161–1170. [Google Scholar] [CrossRef] [PubMed]
- Verma, G.; Marella, A.; Shaquiquzzaman, M.; Akhtar, M.; Rahmat Ali, M.; Alam, M.M. A review exploring biological activities of hydrazones. J. Pharm. Biol. Sci. 2014, 6, 69–80. [Google Scholar]
- Rollas, M.; Küçükgüzel, S.G. Biological Activities of Hydrazone Derivatives. Molecules 2007, 12, 1910–1939. [Google Scholar] [CrossRef] [Green Version]
- Popiołek, Ł.; Stefańska, J.; Kiełczykowska, M.; Musik, I.; Biernasiuk, A.; Malm, A.; Wujec, M. Synthesis, Dissociation Constants, and Antimicrobial Activity of Novel 2,3-Disubstituted-1,3-thiazolidin-4-one Derivatives. J. Heterocycl. Chem. 2015, 53, 393–402. [Google Scholar] [CrossRef]
- Popp, F.D.; Kirby, J.A. Ferrocenylidene Hydrazides. J. Chem. Eng. Data 1963, 8, 604. [Google Scholar] [CrossRef]
- Rector, D.L.; Conder, G.A.; Folz, S.D. Anthelmintic Pyridinyl Acylhydrazones, Method of Use and Compositions. Publication Date: 14 August 1986. Available online: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO1986004582 (accessed on 31 July 2021).
- Palla, G.; Predieri, G.; Domiano, P.; Vignali, C.; Turner, W. Conformational behaviour and E/Z isomerization of N-acyl and N-aroylhydrazones. Tetrahedron 1986, 42, 3649–3654. [Google Scholar] [CrossRef]
- Kumar, P.; Kadyan, K.; Duhan, M.; Sindhu, J.; Singh, V.; Saharan, B.S. Design, synthesis, conformational and molecular docking study of some novel acyl hydrazone based molecular hybrids as antimalarial and antimicrobial agents. Chem. Cent. J. 2017, 11, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, C.; Htan, B.; Ma, C.; Liao, R.-Z.; Gan, Q. Acylhydrazone Switches: E/Z Stability Reversed by Indroduction of Hydrogen Bonds. Eur. J. Org. Chem. 2018, 48, 7046–7050. [Google Scholar] [CrossRef]
- Thota, S.; Rodrigues, D.A.; Pinheiro, P.S.M.; Lima, L.M.; Fraga, C.A.M.; Barreiro, E.J.B. N-Acylhydrazones as drugs. Bioorg. Med. Chem. Lett. 2018, 28, 2797–2806. [Google Scholar] [CrossRef]
- Kress, H.G. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur. J. Pain. 2009, 13, 11–15. [Google Scholar] [CrossRef]
- Karra, R.; Rossing, S.H.; Mohammed, D.; Parmeggiani, L.; Heine, M.; Namnún, O.C. Unmet needs in the management of functional impairment in patients with chronic pain: A multinational survey. Pain Manag. 2021, 11, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.F.; Júnior, W.B.; Moreira, M.S.A.; Costa, F.N.; Monteiro, C.E.S.; Ferreira, F.F.; Barroso, R.C.R.; Noël, F.; Sudo, R.T.; Sudo, G.Z.; et al. Novel Orally Active Analgesic and Anti-Inflammatory Cyclohexyl-N-Acylhydrazone Derivatives. Molecules 2015, 20, 3067–3088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, L.M.; Alves, M.A.; Amaral, D.N. Homologation: A Versatile Molecular Modification Strategy to Drug Discovery. Cur. Top. Med. Chem. 2019, 19, 1734–1750. [Google Scholar] [CrossRef]
- Lima, L.M.; Barreiro, E.J. Bioisosterism: A Useful Strategy for Molecular Modification and Drug Design. Cur. Med. Chem. 2005, 12, 23–49. [Google Scholar] [CrossRef] [PubMed]
- Lima, L.M.; Barreiro, E.J. Beyond Bioisosterism: New Concepts in Drug Discovery. In Comprehensive Medicinal Chemistry. III, 3rd ed.; Elsevier, 2017; eBook; v.1; pp. 186–210. Available online: https://www.elsevier.com/books/comprehensive-medicinal-chemistry-iii/chackalamannil/978-0-12-803200-8 (accessed on 9 August 2021)ISBN 9780128032015.
- Bastos, I.T.S.; Costa, F.N.; Silva, T.F.; Barreiro, E.J.; Lima, L.M.; Braz, D.; Lombardo, G.M.; Punzo, F.; Ferreira, F.F.; Barroso, R.C. A combined experimental and in silico characterization to highlight additional structural features and properties of a potentially new drug. J. Mol. Struct. 2017, 1146, 735–743. [Google Scholar] [CrossRef]
- Available online: https://www.acdlabs.com/products/percepta/ (accessed on 31 July 2021).
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, M.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 0066. [Google Scholar] [CrossRef]
- Abraham, M.H.; Benjelloun-Dakhama, N.; Gola, J.M.R.; Acree, W.E., Jr.; Cain, W.S.; Enrique Cometto-Muniz, J. Solvation descriptors for ferrocene, and the estimation of some physicochemical and biochemical properties. New J. Chem. 2000, 24, 825–829. [Google Scholar] [CrossRef]
- Collier, H.O.; Dinneen, L.C.; Johnson, C.A.; Schneider, C. The abdominal constriction response and its suppression by analgesic drugs in the mouse. Br. J. Pharmacol. Chemother. 1968, 32, 295–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunskaar, S.; Hole, K. The formalin test in mice: Dissociation between inflammatory and non-inflammatory pain. Pain 1987, 30, 103–114. [Google Scholar] [CrossRef]
- Ferrandiz, M.L.; Alcaraz, M.J. Anti-inflammatory activity and inhibition of arachidonic acid metabolism by flavonoids. Agents Actions 1991, 32, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, E.; Stark, A.; Hörig, C. Cyclische Diazoverbindungen, V: Ein Diazoketon mit Dreiringstruktur. Eur. J. In. Chem. 1965, 98, 2509–2515. [Google Scholar] [CrossRef]
- Martin, W.B.; Swett, M.L.R. Isopropylhydrazine Derivatives. US2928875A, 15 March 1960. [Google Scholar]
- Liu, X.H. High Throughput Receptor-Based Virtual Screening under ZINC Database, Synthesis, and Biological Evaluation of Ketol-Acid Reductoisomerase Inhibitors. Chem. Biol. Drug Des. 2010, 75, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Sudo, R.T.; Neto, M.L.; Monteiro, C.E.; Amaral, R.V.; Resende, A.C.; Souza, P.J.; ZapataSudo, G.; Moura, R.S. Antinociceptive effects of hydroalcoholic extract from Euterpe oleracea Mart. (Acai) in a rodent model of acute and neuropathic pain. BMC Complement. Altern. Med. 2015, 15, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendes, T.C.; Raimundo, J.M.; Nascimento-Junior, N.M.; Fraga, C.A.; Barreiro, E.J.; Sudo, R.T.; Zapata-Sudo, G. Sedation and antinociception induced by a new pyrazolo [3,4-b]pyrrolo[3,4-d]pyridine derivative (LASSBio-873) is modulated by activation of muscarinic receptors. Pharmacol. Biochem. Behav. 2009, 94, 70–74. [Google Scholar] [CrossRef]
- Kim, S.H.; Chung, J.M. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain 1992, 50, 355–363. [Google Scholar] [CrossRef]






| Compound | Physicochemical Properties | ||||||
|---|---|---|---|---|---|---|---|
| M.W. g/mol | LogP | LogD4,6 | LogD7,4 | pKa | TPSA | Solubility mg/mL | |
| 1 | 231.30 | 1.95 | 1.93 | 1.95 | 11.5 ± 0.4 5.2 ± 0.8 | 54.35 | 0.18 |
| 2a | 217.27 | 1.48 | 1.46 | 1.48 | 11.5 ± 0.4 5.2 ± 0.8 | 54.35 | 0.25 |
| 2b | 216.28 | 2.78 | 2.78 | 2.78 | 11.5 ± 0.4 | 41.46 | 0.01 |
| 2c | 222.31 | 2.70 | 2.70 | 2.70 | 11.5 ± 0.4 | 69.70 | 0.21 |
| 2d | 324.21 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 2e | 259.35 | 3.05 | 3.02 | 3.05 | 11.5 ± 0.4 3.4 ± 0.4 | 44.70 | 0.27 |
| 3a | 203.24 | 1.23 | 1.21 | 1.23 | 11.5 ± 0.4 5.2 ± 0.4 | 54.35 | 1.31 |
| 3b | 202.25 | 2.09 | 2.09 | 2.09 | 11.5 ± 0.4 | 41.46 | 0.13 |
| 3c | 208.28 | 2.00 | 2.00 | 2.00 | 11.5 ± 0.4 | 69.70 | 0.35 |
| 3d | 310.18 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3e | 245.32 | 2.52 | 2.52 | 2.52 | 11.5 ± 0.4 3.4 ± 0.4 | 44.70 | 0.13 |
| 4a | 189.21 | 0.68 | 0.66 | 0.68 | 11.1 ± 0.5 5.2 ± 0.8 | 54.35 | 1.02 |
| 4b | 188.23 | 1.70 | 1.70 | 1.70 | 11.1 ± 0.5 | 41.46 | 0.12 |
| 4c | 194.25 | 1.64 | 1.64 | 1.64 | 11.1 ± 0.5 | 69.70 | 0.31 |
| 4d | 296.15 | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4e | 231.29 | 2.16 | 2.13 | 2.16 | 11.1 ± 0.5 3.4 ± 0.4 | 44.70 | 0.12 |
| Compound | ADMET Properties | |||||||
|---|---|---|---|---|---|---|---|---|
| Caco-2 (cm/s) | PPB (%) | Vd (L/Kg) | CNS | Foral (%) | HLM | AMES | hErg | |
| 1 | 187 × 10−6 | 81 | 1.6 | −2.45 | 99 | 0.48 | 0.44 | 0.39 |
| 2a | 155 × 10−6 | 70 | 1.5 | −2.30 | 99 | 0.47 | 0.45 | 0.39 |
| 2b | 224 × 10−6 | 84 | 1.9 | −2.14 | 80 | 0.44 | 0.37 | 0.38 |
| 2c | 223 × 10−6 | 85 | 2.0 | −2.10 | 98 | 0.50 | 0.43 | 0.35 |
| 2d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 2e | 229 × 10−6 | 87 | 2.0 | −2.17 | 97 | 0.51 | 0.48 | 0.40 |
| 3a | 135 × 10−6 | 61 | 1.4 | −2.27 | 99 | 0.49 | 0.48 | 0.39 |
| 3b | 198 × 10−6 | 77 | 1.8 | −2.02 | 99 | 0.48 | 0.43 | 0.38 |
| 3c | 193 × 10−6 | 85 | 1.9 | −2.30 | 99 | 0.50 | 0.46 | 0.35 |
| 3d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 3e | 215 × 10−6 | 72 | 1.9 | −1.97 | 99 | 0.49 | 0.48 | 0.39 |
| 4a | 91 × 10−6 | 57 | 1.3 | −2.46 | 99 | 0.46 | 0.55 | 0.37 |
| 4b | 174 × 10−6 | 74 | 1.6 | −2.18 | 96 | 0.47 | 0.58 | 0.37 |
| 4c | 171 × 10−6 | 86 | 1.6 | −2.43 | 99 | 0.49 | 0.57 | 0.34 |
| 4d | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. | N.D. |
| 4e | 200 × 10−6 | 74 | 1.6 | −2.11 | 98 | 0.47 | 0.57 | 0.39 |
| Analgesic Activity (% Inhibition) | |||
|---|---|---|---|
| Compounds | Acetic Acid-Induced Pain | Formalin-Induced Pain | |
| 1st Phase | 2nd Phase | ||
| Indomethacin | N.D. | 23.5 ± 7.9 | 57.4 ± 5.8 ** |
| Dipyrone | 82.5 ± 6.2 ** | N.D. | N.D. |
| LASSBio1514 (1) | 65.7 ± 9.5 ** | 36.9 ± 8.5 | 43.0 ± 5.7 ** |
| 2a | 39.5 ± 3.6 ** | 37.4 ± 4.9 ** | 51.7 ± 6.8 ** |
| 2b | 51.6 ± 8.7 ** | 45.8 ± 12.1 ** | 36.2 ± 2.1 ** |
| 2c | 51.6 ± 8.6 ** | 47.6 ± 12.6 ** | 57.6 ± 8.7 ** |
| 2d | 60.5 ± 7.0 ** | 46.9 ± 3.5 ** | 38.7 ± 5.8 ** |
| 2e | 75.4 ± 8.1 ** | 31.5 ± 13.7 | 63.9 ± 4.5 ** |
| 3a | 66.7 ± 10.4 ** | 15.4 ± 7.3 | 35.5 ± 8.3 * |
| 3b | 94.3 ± 2.0 ** | 9.4 ± 4.1 | 31.8 ± 8.5 |
| 3c | 79.4 ± 3.3 ** | 9.5 ± 6.4 | 43.4 ± 2.6 ** |
| 3d | 65.7 ± 6.3 ** | 29.6 ± 8.6 | 18.4 ± 11.7 |
| 3e | 54.0 ± 5.2 ** | 5.7 ± 4.7 | 22.4 ± 9.5 |
| 4a | 71.8 ± 0.8 ** | 76.3 ± 3.3 ** | 34.9 ± 3.8 * |
| 4b | 70.8 ± 1.9 ** | 36.1 ± 1.8 | 13.1 ± 4.9 |
| 4c | 77.8 ± 7.6 ** | 9.5 ± 6.5 | 0.6 ± 6.1 |
| 4d | 72.3 ± 0.9 ** | 42.3 ± 12.9 * | 43.8 ± 7.9 ** |
| 4e | 51.1 ± 3.8 ** | 64.9 ± 6.2 ** | 37.1 ± 11.7 ** |
| Carrageenan Induced Peritonitis | |
|---|---|
| Compounds | % Leukocyte Infiltration Inhibition |
| Indomethacin | 65.06 ± 4.0 ** |
| LASSBio1514 (1) | 81.9 ± 2.2 ** |
| 2a | 42.7 ± 2.1 ** |
| 2b | 54.9 ± 5.3 ** |
| 2c | 47.6 ± 8.9 ** |
| 2d | 44.2 ± 3.2 ** |
| 2e | 57.7 ± 7.1 ** |
| 3a | 45.6 ± 5.6 ** |
| 3b | 72.9 ± 5.8 ** |
| 3c | 61.4 ± 4.2 ** |
| 3d | 58.4 ± 4.2 ** |
| 3e | 62.1 ± 7.9 ** |
| 4a | 41.6 ± 3.4 ** |
| 4b | 31.7 ± 3.5 ** |
| 4c | 55.4 ± 3.9 ** |
| 4d | 35.5 ± 3.8 ** |
| 4e | 49.2 ± 4.3 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lima, L.M.; da Silva, T.F.; da Silva Monteiro, C.E.; Aparecida-Silva, C.; Bispo Júnior, W.; de Queiroz, A.C.; Alexandre-Moreira, M.S.; Zapata-Sudo, G.; Barreiro, E.J. Design and Synthesis In Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514. Molecules 2021, 26, 4828. https://doi.org/10.3390/molecules26164828
Lima LM, da Silva TF, da Silva Monteiro CE, Aparecida-Silva C, Bispo Júnior W, de Queiroz AC, Alexandre-Moreira MS, Zapata-Sudo G, Barreiro EJ. Design and Synthesis In Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514. Molecules. 2021; 26(16):4828. https://doi.org/10.3390/molecules26164828
Chicago/Turabian StyleLima, Lidia Moreira, Tiago Fernandes da Silva, Carlos Eduardo da Silva Monteiro, Cristiane Aparecida-Silva, Walfrido Bispo Júnior, Aline Cavalcanti de Queiroz, Magna Suzana Alexandre-Moreira, Gisele Zapata-Sudo, and Eliezer J. Barreiro. 2021. "Design and Synthesis In Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514" Molecules 26, no. 16: 4828. https://doi.org/10.3390/molecules26164828
APA StyleLima, L. M., da Silva, T. F., da Silva Monteiro, C. E., Aparecida-Silva, C., Bispo Júnior, W., de Queiroz, A. C., Alexandre-Moreira, M. S., Zapata-Sudo, G., & Barreiro, E. J. (2021). Design and Synthesis In Silico Drug-like Prediction and Pharmacological Evaluation of Cyclopolymethylenic Homologous of LASSBio-1514. Molecules, 26(16), 4828. https://doi.org/10.3390/molecules26164828