Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials
Abstract
:1. Introduction
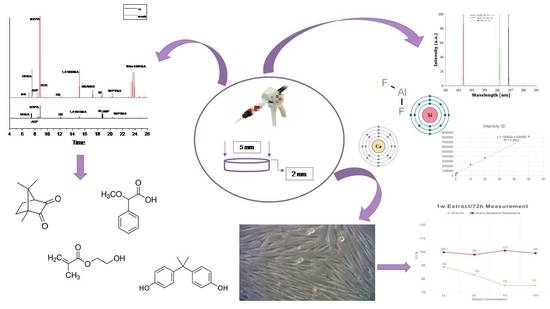
2. Results
2.1. Organic Eluates Determination in Methanol and Artificial Saliva by Using GC/MS
2.2. Ions Release in Deionized Water by Using ICP–OES
2.3. Cell Viability
3. Discussion
4. Materials and Methods
4.1. Materials and Specimens Preparation for GC/MS and ICP–OES Analysis
4.2. Monomer Elution Evaluation
4.3. Specimens in Methanol
4.3.1. Specimens in Artificial Saliva
4.3.2. Separation by Gas Chromatography and Mass Spectrometric Detection
4.4. Ion Release
4.5. Cell Viability Experiment
4.5.1. Restorative Materials Extracts
4.5.2. Cell Culture
4.5.3. Cell Viability
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ruyter, I.E. Monomer systems and polymerization. In Posterior Composite Resin Dental Restorative Materials; Vanherle, G., Smith, D.C., Eds.; Peter Szulc Publishing Co.: Amsterdam, The Netherlands, 1985; pp. 109–135. [Google Scholar]
- Geurtsen, W.; Spahl, W.; Leyhausen, G. Variability of cytotoxicity and leaching of substances from four light-curing pit and fissure sealants. J. Biomed. Mater. Res. 1999, 44, 73–77. [Google Scholar] [CrossRef]
- Moon, H.J.; Lim, B.S.; Lee, Y.K.; Kim, C.W. Determination of residual monomers in dental pit and fissure sealants using food/oral simulating fluids. Bull. Korean Chem. Soc. 2000, 21, 1115–1118. [Google Scholar]
- Michelsen, V.B.; Moe, G.; Skålevik, R.; Jensen, E.; Lygre, H. Quantification of organic eluates from polymerized resin-based dental restorative materials by use of GC/MS. J. Chromatogr. B 2007, 850, 83–91. [Google Scholar] [CrossRef]
- Seiss, M.; Langer, C.; Hickel, R.; Reichl, F.X. Quantitative determination of TEGDMA, BHT, and DMABEE in eluates from polymerized resin-based dental restorative materials by use of GC/MS. Arch. Toxicol. 2009, 83, 1109–1115. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, J.L. Elution of leachable components from composites. J. Oral Rehabil. 1994, 21, 441–452. [Google Scholar] [CrossRef] [PubMed]
- Sideridou, I.D.; Achilias, D.S. Elution study of unreacted Bis-GMA, TEGDMA, UDMA, and Bis-EMA from light-cured dental resins and resin composites using HPLC. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 74, 617–626. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.S.; Darmani, H.; Elbetieha, A.M. Leached components from dental composites and their effects on fertility of female mice. Eur. J. Oral Sci. 2004, 112, 267–272. [Google Scholar] [CrossRef]
- Becher, R.; Kopperud, H.M.; Al, R.H.; Samuelsen, J.T.; Morisbak, E.; Dahlman, H.J.; Lilleaas, E.M.; Dahl, J.E. Pattern of cell death after in vitro exposure to GDMA, TEGDMA, HEMA and two compomer extracts. Dent. Mater. 2006, 22, 630–640. [Google Scholar] [CrossRef] [PubMed]
- Bouillaguet, S.; Wataha, J.C.; Hanks, C.T.; Ciucchi, B.; Holz, J. In vitro cytotoxicity and dentin permeability of HEMA. J. Endod. 1996, 22, 244–248. [Google Scholar] [CrossRef]
- Geurtsen, W. Biocompatibility of resin-modified filling materials. Crit. Rev. Oral Biol. Med. 2000, 11, 333–355. [Google Scholar] [CrossRef] [PubMed]
- Heil, J.; Reifferscheid, G.; Waldmann, P.; Leyhausen, G.; Geurtsen, W. Genotoxicity of dental materials. Mutat. Res. Genet. Toxicol. 1996, 368, 181–194. [Google Scholar] [CrossRef]
- Schweikl, H.; Altmannberger, I.; Hanser, N.; Hiller, K.-A.A.; Bolay, C.; Brockhoff, G.; Spagnuolo, G.; Galler, K.; Schmalz, G. The effect of triethylene glycol dimethacrylate on the cell cycle of mammalian cells. Biomaterials 2005, 26, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Hensten-Pettersen, A. Skin and mucosal reactions associated with dental materials. Eur. J. Oral Sci. 1998, 106, 707–712. [Google Scholar] [PubMed]
- Lewis, J.B.; Rueggeberg, F.A.; Lapp, C.A.; Ergle, J.W.; Schuster, G.S. Identification and characterization of estrogen-like components in commercial resin-based dental restorative materials. Clin. Oral Investig. 1999, 3, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, V.B.; Lygre, H.; Skålevik, R.; Tveit, A.B.; Solheim, E. Identification of organic eluates from four polymer-based dental filling materials. Eur. J. Oral Sci. 2003, 111, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Michelsen, V.B.; Moe, G.; Strøm, M.B.; Jensen, E.; Lygre, H. Quantitative analysis of TEGDMA and HEMA eluted into saliva from two dental composites by use of GC/MS and tailor-made internal standards. Dent. Mater. 2008, 24, 724–731. [Google Scholar] [CrossRef] [PubMed]
- Polydorou, O. Elution of Substances from Dental Composite Materials. In Dental Composite Materials for Direct Restorations; Miletic, V., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2018; pp. 179–195. ISBN 978-3-319-60961-4. [Google Scholar]
- Kerezoudi, C.; Gogos, C.; Samanidou, V.; Tziafas, D.; Palaghias, G. Evaluation of monomer leaching from a resin cement through dentin by a novel model. Dent. Mater. 2016, 32, e297–e305. [Google Scholar] [CrossRef]
- Van Landuyt, K.L.; Nawrot, T.; Geebelen, B.; De Munck, J.; Snauwaert, J.; Yoshihara, K.; Scheers, H.; Godderis, L.; Hoet, P.; Van Meerbeek, B. How much do resin-based dental materials release? A meta-analytical approach. Dent. Mater. 2011, 27, 723–747. [Google Scholar] [CrossRef]
- Durner, J.; Schrickel, K.; Watts, D.C.; Ilie, N. Determination of homologous distributions of bisEMAdimethacrylates in bulk-fill resin-composites by GC-MS. Dent. Mater. 2015, 31, 473–480. [Google Scholar] [CrossRef]
- Spahl, W.; Budzikiewicz, H.; Geurtsen, W. Determination of leachable components from four commercial dental composites by gas and liquid chromatography/mass spectrometry. J. Dent. 1998, 26, 137–145. [Google Scholar] [CrossRef]
- Jandt, K.D.; Sigusch, B.W. Future perspectives of resin-based dental materials. Dent. Mater. 2009, 25, 1001–1006. [Google Scholar] [CrossRef]
- Chiari, M.D.S.; Rodrigues, M.C.; Xavier, T.A.; De Souza, E.M.N.; Arana-Chavez, V.E.; Braga, R.R. Mechanical properties and ion release from bioactive restorative composites containing glass fillers and calcium phosphate nano-structured particles. Dent. Mater. 2015, 31, 726–733. [Google Scholar] [CrossRef]
- Chen, M.-H. Update on Dental Nanocomposites. J. Dent. Res. 2010, 89, 549–560. [Google Scholar] [CrossRef]
- Ferracane, J.L. Resin composite—State of the art. Dent. Mater. 2011, 27, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, A.; Chosa, N.; Kyakumoto, S.; Yokota, S.; Kamo, M.; Noda, M.; Ishisaki, A. Water-soluble factors eluated from Surface pre-reacted glass-ionomer filler promote osteoblastic differentiation of human mesenchymal stem cells. Mol. Med. Rep. 2018, 17, 3448–3454. [Google Scholar] [CrossRef] [PubMed]
- Ikemura, K.; Tay, F.R.; Endo, T.; Pashley, D.H. A Review of Chemical-approach and Ultramorphological Studies on the Development of Fluoride-releasing Dental Adhesives Comprising New Pre-Reacted Glass Ionomer (PRG) Fillers. Dent. Mater. J. 2008, 27, 315–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, S.; Iijima, M.; Hashimoto, M.; Tsukamoto, N.; Mizoguchi, I.; Saito, T. Effects of surface pre-reacted glass-ionomer fillers on mineral induction by phosphoprotein. J. Dent. 2011, 39, 72–79. [Google Scholar] [CrossRef]
- Vouzara, T.; Roussou, K.; Nikolaidis, A.K.; Tolidis, K.; Koulaouzidou, E.A. Organic Eluates Derived from Intermediate Restorative Dental Materials. Molecules 2020, 25, 1593. [Google Scholar] [CrossRef] [Green Version]
- Jun, S.-K.; Lee, J.-H.; Lee, H.-H. The Biomineralization of a Bioactive Glass-Incorporated Light-Curable Pulp Capping Material Using Human Dental Pulp Stem Cells. Biomed. Res. Int. 2017, 2017, 2495282. [Google Scholar] [CrossRef] [Green Version]
- Bijelic-Donova, J.; Garoushi, S.; Vallittu, P.K.; Lassila, L.V.J. Mechanical properties, fracture resistance, and fatigue limits of short fiber reinforced dental composite resin. J. Prosthet. Dent. 2016, 115, 95–102. [Google Scholar] [CrossRef]
- Bijelic-Donova, J.; Garoushi, S.; Lassila, L.V.J.; Keulemans, F.; Vallittu, P.K. Mechanical and structural characterization of discontinuous fiber-reinforced dental resin composite. J. Dent. 2016, 52, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Małkiewicz, K.; Wychowański, P.; Olkowska-Truchanowicz, J.; Tykarska, M.; Wilczko, M.; Czerwiński, M.; Owoc, A. Uncompleted Polymerization and Cytotoxicity of Dental Restorative Materials as Potential Health Risk Factors. Ann. Agric. Environ. Med. 2017, 24, 618–623. [Google Scholar] [CrossRef] [PubMed]
- Durner, J.; Spahl, W.; Zaspel, J.; Schweikl, H.; Hickel, R.; Reichl, F.X. Eluted substances from unpolymerized and polymerized dental restorative materials and their Nernst partition coefficient. Dent. Mater. 2010, 26, 91–99. [Google Scholar] [CrossRef]
- Rothmund, L.; Shehata, M.; Van Landuyt, K.L.; Schweikl, H.; Carell, T.; Geurtsen, W.; Hellwig, E.; Hickel, R.; Reichl, F.X.; Högg, C. Release and protein binding of components from resin based composites in native saliva and other extraction media. Dent. Mater. 2015, 31, 496–504. [Google Scholar] [CrossRef]
- Sevkusic, M.; Schuster, L.; Rothmund, L.; Dettinger, K.; Maier, M.; Hickel, R.; Van Landhuyt, K.L.; Durner, J.; Högg, C.; Reichl, F.X. The elution and breakdown behavior of constituents from various light-cured composites. Dent. Mater. 2014, 30, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Alshali, R.Z.; Salim, N.A.; Sung, R.; Satterthwaite, J.D.; Silikas, N. Analysis of long-term monomer elution from bulk-fill and conventional resin-composites using high performance liquid chromatography. Dent. Mater. 2015, 31, 1587–1598. [Google Scholar] [CrossRef]
- Finer, Y.; Jaffer, F.; Santerre, J.P. Mutual influence of cholesterol esterase and pseudocholinesterase on the biodegradation of dental composites. Biomaterials 2004, 25, 1787–1793. [Google Scholar] [CrossRef]
- Finer, Y.; Santerre, J.P. Salivary esterase activity and its association with the biodegradation of dental composites. J. Dent. Res. 2004, 83, 22–26. [Google Scholar] [CrossRef]
- Lyche, J.L. Phthalates. In Reproductive and Developmental Toxicology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 637–655. [Google Scholar]
- Ellis, B.; AI-Nakash, S.; Lamb, D.J.; McDonald, M.P. A study of the composition and diffusion characteristics of a soft liner. J. Dent. 1979, 7, 133–140. [Google Scholar] [CrossRef]
- Braden, M.; Causton, B.E. Tissue Conditioners: III. Water Immersion Characteristics. J. Dent. Res. 1971, 50, 1544–1547. [Google Scholar] [CrossRef]
- Wallace, D.R. Dibutyl Phthalate*. In Encyclopedia of Toxicology; Elsevier: Amsterdam, The Netherlands, 2005; pp. 1–2. [Google Scholar]
- Peijnenburg, W.J.G.M. Phthalates. In Encyclopedia of Ecology; Elsevier: Amsterdam, The Netherlands, 2008; pp. 2733–2738. [Google Scholar]
- Kawahara, T.; Nomura, Y.; Tanaka, N.; Teshima, W.; Okazaki, M.; Shintani, H. Leachability of plasticizer and residual monomer from commercial temporary restorative resins. J. Dent. 2004, 32, 277–283. [Google Scholar] [CrossRef]
- Chaves, C.D.A.L.; MacHado, A.L.; Carlos, I.Z.; Giampaolo, E.T.; Pavarina, A.C.; Vergani, C.E. Cytotoxicity of monomers, plasticizer and degradation by-products released from dental hard chairside reline resins. Dent. Mater. 2010, 26, 1017–1023. [Google Scholar] [CrossRef]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Shultz, V.D.; Phillips, S.; Sar, M.; Foster, P.M.; Gaido, K.W. Altered gene profiles in fetal rat testes after in utero exposure to di(n-butyl) phthalate. Toxicol. Sci. 2001, 64, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Mylchreest, E.; Wallace, D.G.; Cattley, R.C.; Foster, P.M. Dose-dependent alterations in androgen-regulated male reproductive development in rats exposed to Di(n-butyl) phthalate during late gestation. Toxicol. Sci. 2000, 55, 143–151. [Google Scholar] [CrossRef] [Green Version]
- Nair, M.G.; Burke, B.A. A new fatty acid methyl ester and other biologically active compounds from Aspergillus niger. Phytochemistry 1988, 27, 3169–3173. [Google Scholar] [CrossRef]
- Ichiba, T.; Murashi, T.; Ohtsuka, T.; Masuko, M. Fungicidal Activities of α-Methoxyphenylacetic Acid Derivatives. J. Pestic. Sci. 2002, 27, 118–126. [Google Scholar] [CrossRef] [Green Version]
- Kaur, M.; Srivastava, A.K. Photopolymerization: A Review. J. Macromol. Sci. Part C Polym. Rev. 2002, 42, 481–512. [Google Scholar] [CrossRef]
- Jędrzejewska, B. Factors affecting the TMPTA radical polymerization photoinitiated by phenyltrialkylborates paired with tri-cationic hemicyanine dye. Kinetic studies. Colloid Polym. Sci. 2013, 291, 2225–2236. [Google Scholar] [CrossRef] [Green Version]
- Frick, E.; Ernst, H.A.; Voll, D.; Wolf, T.J.A.; Unterreiner, A.-N.; Barner-Kowollik, C. Studying the polymerization initiation efficiency of acetophenone-type initiators via PLP-ESI-MS and femtosecond spectroscopy. Polym. Chem. 2014, 5, 5053–5068. [Google Scholar] [CrossRef] [Green Version]
- Fugolin, A.P.; Dobson, A.; Ferracane, J.L.; Pfeifer, C.S. Effect of residual solvent on performance of acrylamide-containing dental materials. Dent. Mater. 2019, 35, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhong, M.; Johnson, J.A. Light-Controlled Radical Polymerization: Mechanisms, Methods, and Applications. Chem. Rev. 2016, 116, 10167–10211. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Stansbury, J.W. RAFT-mediated control of nanogel structure and reactivity: Chemical, physical and mechanical properties of monomer-dispersed nanogel compositions. Dent. Mater. 2014, 30, 1252–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moldoveanu, S.C. Pyrolysis of Peroxy Compounds. In Pyrolysis of Organic Molecules; Elsevier: Amsterdan, The Netherlands, 2019; pp. 311–319. [Google Scholar]
- Su, W.-F. Principles of Polymer Design and Synthesis; Lecture Notes in Chemistry; Springer Berlin Heidelberg: Berlin, Heidelberg, Germany, 2013; Volume 82, ISBN 978-3-642-38729-6. [Google Scholar]
- Antonucci, J.M.; Peckoo, R.J.; Schruhl, C.; Toth, E.E. Slow-acting Amine Polymerization Accelerators. Para-dimethylaminobenzoic Acid and Its Ethyl Ester. J. Dent. Res. 1981, 60, 1325–1331. [Google Scholar] [CrossRef]
- Jan, C.M.; Nomura, Y.; Urabe, H.; Okazaki, M.; Shintani, H. The relationship between leachability of polymerization initiator and degree of conversion of visible light-cured resin. J. Biomed. Mater. Res. 2001, 58, 42–46. [Google Scholar] [CrossRef]
- Chang, M.-C.; Lin, L.-D.; Wu, M.-T.; Chan, C.-P.; Chang, H.-H.; Lee, M.-S.; Sun, T.-Y.; Jeng, P.-Y.; Yeung, S.-Y.; Lin, H.-J.; et al. Effects of Camphorquinone on Cytotoxicity, Cell Cycle Regulation and Prostaglandin E2 Production of Dental Pulp Cells: Role of ROS, ATM/Chk2, MEK/ERK and Hemeoxygenase-1. PLoS ONE 2015, 10, e0143663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, F.; McCabe, A.W.G.W. Applied Dental Materials, 9th ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2013. [Google Scholar]
- Geurtsen, W.; Spahl, W.; Leyhausen, G. Residual monomer/additive release and variability in cytotoxicity of light-curing glass-ionomer cements and compomers. J. Dent. Res. 1998, 77, 2012–2019. [Google Scholar] [CrossRef]
- Nilsen, B.W.; Jensen, E.; Örtengren, U.; Michelsen, V.B. Analysis of organic components in resin-modified pulp capping materials: Critical considerations. Eur. J. Oral Sci. 2017. [Google Scholar] [CrossRef]
- Schweikl, H.; Schmalz, G.; Spruss, T. The Induction of Micronuclei in vitro by Unpolymerized Resin Monomers. J. Dent. Res. 2001, 80, 1615–1620. [Google Scholar] [CrossRef]
- Scully, C. Occupational hazards. In Scully’s Medical Problems in Dentistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 713–729. [Google Scholar]
- Nocca, G.; Martorana, G.E.; De Sole, P.; De Palma, F.; Callà, C.; Corsale, P.; Antenucci, M.; Gambarini, G.; Chimenti, C.; Giardina, B.; et al. Effects of 1,4-butanediol dimethacrylate and urethane dimethacrylate on HL-60 cell metabolism. Eur. J. Oral Sci. 2009, 117, 175–181. [Google Scholar] [CrossRef]
- Silva, E.M.; Da Miragaya, L.; Noronha-Filho, J.D.; Amaral, C.M.; Poskus, L.T.; Guimarães, J.G.A. Characterization of an experimental resin composite organic matrix based on a tri-functional methacrylate monomer. Dent. Mater. J. 2016, 35, 159–165. [Google Scholar] [CrossRef] [Green Version]
- Wada, K.; Ikeda, E.; Wada, J.; Inoue, G.; Miyasaka, M.; Miyashin, M. Wear characteristics of trimethylolpropane trimethacrylate filler-containing resins for the full crown restoration of primary molars. Dent. Mater. J. 2016, 35, 585–593. [Google Scholar] [CrossRef] [Green Version]
- Konieczna, A.; Rutkowska, A.; Rachoń, D. Health risk of exposure to Bisphenol A (BPA). Roczniki Państwowego Zakładu Higieny 2015, 66, 5–11. [Google Scholar]
- Atkinson, J.C.; Diamond, F.; Eichmiller, F.; Selwitz, R.; Jones, G. Stability of bisphenol A, triethylene-glycol dimethacrylate, and bisphenol A dimethacrylate in whole saliva. Dent. Mater. 2002, 18, 128–135. [Google Scholar] [CrossRef]
- Berge, T.L.L.; Lygre, G.B.; Jönsson, B.A.G.; Lindh, C.H.; Björkman, L. Bisphenol A concentration in human saliva related to dental polymer-based fillings. Clin. Oral Investig. 2017, 21, 1–8. [Google Scholar] [CrossRef]
- Terasaka, H.; Kadoma, Y.; Sakagami, H.; Fujisawa, S. Cytotoxicity and apoptosis-inducing activity of bisphenol A and hydroquinone in HL-60 cells. Anticancer Res. 2005, 25, 2241–2247. [Google Scholar]
- Koulaouzidou, E.A.; Roussou, K.; Sidiropoulos, K.; Nikolaidis, A.; Kolokuris, I.; Tsakalof, A.; Tsitsimpikou, C.; Kouretas, D. Investigation of the chemical profile and cytotoxicity evaluation of organic components eluted from pit and fissure sealants. Food Chem. Toxicol. 2018, 120, 536–543. [Google Scholar] [CrossRef]
- Suzuki, K.; Ishikawa, K.; Sugiyama, K.; Furuta, H.; Nishimura, F. Content and release of bisphenol A from polycarbonate dental products. Dent. Mater. J. 2000, 19, 389–395. [Google Scholar] [CrossRef]
- Manabe, A.; Kaneko, S.; Numazawa, S.; Itoh, K.; Inoue, M.; Hisamitsu, H.; Sasa, R.; Yoshida, T. Detection of BPA in Dental Materials by Gas Chromatography-Mass Spectrometry. Dent. Mater. J. 2000, 19, 75–86. [Google Scholar] [CrossRef] [Green Version]
- Pulgar, R.; Olea-Serrano, M.F.; Novillo-Fertrell, A.; Rivas, A.; Pazos, P.; Pedraza, V.; Navajas, J.M.; Olea, N. Determination of bisphenol A and related aromatic compounds released from Bis-GMA-based composites and sealants by high performance liquid chromatography. Environ. Health Perspect. 2000, 108, 21–27. [Google Scholar] [CrossRef]
- Olea, N.; Pulgar, R.; Pérez, P.; Olea-Serrano, F.; Rivas, A.; Novillo-Fertrell, A.; Pedraza, V.; Soto, A.M.; Sonnenschein, C. Estrogenicity of resin-based composites and sealants used in dentistry. Environ. Health Perspect. 1996, 104, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Yamaki, K.; Taneda, S.; Yanagisawa, R.; Inoue, K.I.; Takano, H.; Yoshino, S. Enhancement of allergic responses in vivo and in vitro by butylated hydroxytoluene. Toxicol. Appl. Pharmacol. 2007, 223, 164–172. [Google Scholar] [CrossRef]
- Wang, C.; Li, S.-J.; Wu, Z.-Q.; Xu, J.-J.; Chen, H.-Y.; Xia, X.-H. Study on the kinetics of homogeneous enzyme reactions in a micro/nanofluidics device. Lab. Chip 2010, 10, 639–646. [Google Scholar] [CrossRef]
- Weir, M.D.; Chow, L.C.; Xu, H.H.K. Remineralization of Demineralized Enamel via Calcium Phosphate Nanocomposite. J. Dent. Res. 2012, 91, 979–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besinis, A.; van Noort, R.; Martin, N. Remineralization potential of fully demineralized dentin infiltrated with silica and hydroxyapatite nanoparticles. Dent. Mater. 2014, 30, 249–262. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.C.; Natale, L.C.; Arana-Chaves, V.E.; Braga, R.R. Calcium and phosphate release from resin-based materials containing different calcium orthophosphate nanoparticles. J. Biomed. Mater. Res. Part B Appl. Biomater. 2015, 103, 1670–1678. [Google Scholar] [CrossRef]
- Narayana, S.S.; Deepa, V.K.; Ahamed, S.; Sathish, E.S.; Meyappan, R.; Satheesh Kumar, K.S. Remineralization efficiency of bioactive glass on artificially induced carious lesion an in-vitro study. J. Indian Soc. Pedod. Prev. Dent. 2014, 32, 19–25. [Google Scholar]
- Alania, Y.; Chiari, M.D.S.; Rodrigues, M.C.; Arana-Chavez, V.E.; Bressiani, A.H.A.; Vichi, F.M.; Braga, R.R. Bioactive composites containing TEGDMA-functionalized calcium phosphate particles: Degree of conversion, fracture strength and ion release evaluation. Dent. Mater. 2016, 32, e374–e381. [Google Scholar] [CrossRef]
- Natale, L.C.; Rodrigues, M.C.; Alania, Y.; Chiari, M.D.S.; Boaro, L.C.C.; Cotrim, M.; Vega, O.; Braga, R.R. Mechanical characterization and ion release of bioactive dental composites containing calcium phosphate particles. J. Mech. Behav. Biomed. Mater. 2018, 84, 161–167. [Google Scholar] [CrossRef]
- Gregson, K.; Beiswanger, A.J.; Platt, J. The impact of sorption, buffering, and proteins on leaching of organic and inorganic substances from dental resin core material. J. Biomed. Mater. Res. Part A 2008, 84, 256–264. [Google Scholar] [CrossRef]
- Singla, T.; Pandit, I.K.; Srivastava, N.; Gugnani, N.; Gupta, M. An evaluation of microleakage of various glass ionomer based restorative materials in deciduous and permanent teeth: An in vitro study. Saudi Dent. J. 2012, 24, 35–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farooq, I.; Ali, S.; Al-Saleh, S.; AlHamdan, E.M.; AlRefeai, M.H.; Abduljabbar, T.; Vohra, F. Synergistic Effect of Bioactive Inorganic Fillers in Enhancing Properties of Dentin Adhesives—A Review. Polymers 2021, 13, 2169. [Google Scholar] [CrossRef]
- Nicholson, J.W.; Czarnecka, B. The release of ions by compomers under neutral and acidic conditions. J. Oral Rehabil. 2004, 31, 665–670. [Google Scholar] [CrossRef]
- Czarnecka, B.; Nicholson, J.W. Ion release by resin-modified glass-ionomer cements into water and lactic acid solutions. J. Dent. 2006, 34, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Czarnecka, B.; Limanowska-Shaw, H.; Nicholson, J.W. Buffering and ion-release by a glass-ionomer cement under near-neutral and acidic conditions. Biomaterials 2002, 23, 2783–2788. [Google Scholar] [CrossRef]
- Tiskaya, M.; Al-eesa, N.A.; Wong, F.S.L.; Hill, R.G. Characterization of the bioactivity of two commercial composites. Dent. Mater. 2019, 35, 1757–1768. [Google Scholar] [CrossRef] [PubMed]
- Moody, G.H.; Southam, J.C.; Buchan, S.A.; Farmer, J.G. Aluminium leaching and fluoride. Br. Dent. J. 1990, 169, 47–50. [Google Scholar] [CrossRef]
- Miller, R.G.; Kopfler, F.C.; Kelty, K.C.; Stober, J.A.; Ulmer, N.S. The Occurrence of Aluminum in Drinking Water *. J. Am. Water Works Assoc. 1984, 76, 84–91. [Google Scholar] [CrossRef]
- Martin, K.R. Silicon: The Health Benefits of a Metalloid. In Interrelations between essential metal ions and human diseases; Springer: Berlin/Heidelberg, Germany, 2013; pp. 451–473. [Google Scholar]
- Exley, C.; Schneider, C.; Doucet, F.J. The reaction of aluminium with silicic acid in acidic solution: An important mechanism in controlling the biological availability of aluminium? Coord. Chem. Rev. 2002, 228, 127–135. [Google Scholar] [CrossRef]
- Mulder, R.; Anderson-Small, C. Ion release of chitosan and nanodiamond modified glass ionomer restorative cements. Clin. Cosmet. Investig. Dent. 2019, 11, 313–320. [Google Scholar] [CrossRef] [Green Version]
- Noorani, T.Y.; Luddin, N.; Rahman, I.A.; Masudi, S.M. In vitro cytotoxicity evaluation of novel nano-hydroxyapatite-silica incorporated glass ionomer cement. J. Clin. Diagnostic Res. 2017, 11, ZC105–ZC109. [Google Scholar]
- Cosgun, A.; Bolgul, B.; Duran, N. In vitro investigation of antimicrobial effects, nanohardness, and cytotoxicity of different glass ionomer restorative materials in dentistry. Niger. J. Clin. Pract. 2019, 22, 422–431. [Google Scholar]
- Lan, W.-H.; Lan, W.-C.; Wang, T.-M.; Lee, Y.-L.; Tseng, W.-Y.; Lin, C.-P.; Jeng, J.-H.; Chang, M.-C. Cytotoxicity of conventional and modified glass ionomer cements. Oper. Dent. 2003, 28, 251–259. [Google Scholar]
- López-García, S.; Pecci-Lloret, M.P.; Pecci-Lloret, M.R.; Oñate-Sánchez, R.E.; García-Bernal, D.; Castelo-Baz, P.; Rodríguez-Lozano, F.J.; Guerrero-Gironés, J. In Vitro Evaluation of the Biological Effects of ACTIVA Kids BioACTIVE Restorative, Ionolux, and Riva Light Cure on Human Dental Pulp Stem Cells. Materials 2019, 12, 3694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abidin, R.M.Z.; Luddin, N.; Omar, N.S.; Ahmed, H.M.A. Cytotoxicity of fast-set conventional and resin-modified glass ionomer cement polymerized at different times on SHED. J. Clin. Pediatr. Dent. 2015, 39, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Selimović-Dragaš, M.; Huseinbegović, A.; Kobašlija, S.; Hatibović-Kofman, Š. A comparison of the in vitro cytotoxicity of conventional and resin modified glass ionomer cements. Bosn. J. Basic Med. Sci. 2012, 12, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, D.; Zhao, J.; Park, J.G. A novel light-cured glass-ionomer system for improved dental restoratives. J. Mater. Sci. Mater. Med. 2007, 18, 1907–1916. [Google Scholar] [CrossRef]
- Papazisis, K.T.; Geromichalos, G.D.; Dimitriadis, K.A.; Kortsaris, A.H. Optimization of the sulforhodamine B colorimetric assay. J. Immunol. Methods. 1997, 208, 151–158. [Google Scholar] [CrossRef]
- Orellana, E.; Kasinski, A. Sulforhodamine B (SRB) Assay in Cell Culture to Investigate Cell Proliferation. Bio-Protocol 2016, 6, e1984. [Google Scholar] [CrossRef] [Green Version]







| Eluate | Retention Time (RT) | Abbreviation | Molecular Formula | Compound Name | Molecular Weight | Characteristic ions, m/z | Chemical Structure |
|---|---|---|---|---|---|---|---|
| 1 | 7.08 | MS | C9H10 | α-Methylstyrene | 118 | 118, 103, 78, 115 |  |
| 2 | 7.55 | HEMA | C6H10O3 | 2-Hydroxyethyl methacrylate | 130 | 69, 87 |  |
| 3 | 8.51 | ACP | C8H8O | Acetophenone | 120 | 105, 77, 120, 51 |  |
| 4 | 8.82 | MOPA | C9H10O3 | Methoxyphenyl acetic acid | 166 | 121, 77, 51, 78 |  |
| 5 | 8.87 | MCE | C10H14O | Methyl cumyl ether | 150 | 135, 91, 77, 73, 136 |  |
| 6 | 10.71 | MeHQ | C7H8O2 | Mequinol | 124 | 109, 124, 81 |  |
| 7 | 12.14 | CQ | C10H14O2 | Camphorquinone | 166 | 95, 69, 83 |  |
| 8 | 14.77 | BHT | C15H24O | Butylated hydroxytoluene | 220 | 205, 220, 57 |  |
| 9 | 15.12 | 1,4-BDDMA | C12H18O4 | 1,4-Butylene glycol dimethacrylate | 226 | 69, 55, 112 |  |
| 10 | 17.23 | DMABEE | C11H15O2N | Ethyl 4-(dimethylamino) benzoate | 193 | 148, 193, 164 |  |
| 11 | 18.69 | IS | C8H10O2N4 | Caffeine | 194 | 194, 109 |  |
| 12 | 18.91 | DBP | C16H22O4 | Dibutyl phtalate | 278 | 149, 150, 57 |  |
| 13 | 20.34 | TMPTMA | C18H26O6 | Trimethylolpropane trimethacrylate | 338 | 69, 253 |  |
| 14 | 21.92 | BPA | C15H16O2 | Bisphenol A | 228 | 213, 119 |  |
| 15 | 22.79 | TPSb | C18H15Sb | Triphenylstibine | 352 | 198, 154, 200 |  |
| Organic Substances Detected in Artificial Saliva | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||||||||||||||
| 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | |
| Activa Bioactive Restorative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||||
| Ena HRi | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||
| Enamel Plus HRiBioFunction | √ | √ | √ | √ | ||||||||||||||||||||||||||
| GC Fuji II LC | √ | √ | √ | √ | √ | |||||||||||||||||||||||||
| GC Fuji IX | √ | √ | ||||||||||||||||||||||||||||
| Organic Substances Detected in Methanol | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | ||||||||||||||||
| 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | |
| Activa Bioactive Restorative | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||
| Ena HRi | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | ||||||||||||||
| Enamel Plus HRi BioFunction | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||||||||||||||
| GC Fuji II LC | √ | √ | √ | √ | √ | √ | ||||||||||||||||||||||||
| GC Fuji IX | √ | √ | ||||||||||||||||||||||||||||
| Activa Bioactive Restorative | Ena HRi | Enamel Plus Hri BioFunction | GC Fuji II LC | |||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | |
| MS | ||||||||
| ACP | 4.49 (9.32) | |||||||
| MCE | ||||||||
| BHT | ||||||||
| BPA | ||||||||
| 1,4-BDDMA | 5.24 (100.11) | |||||||
| CQ | 0.24 (9.91) | 19.45 (94.94) * | 1,24 (31.86) ** | 1.30 (3.15) * | 0.21 (1.60) | |||
| DMABEE | ||||||||
| DBP | 69.96 (13.05) * | 32.51 (76.07) ** | 131.76 (50.7) * | 43.79 (223.36) ** | 25.80 (30.66) * | |||
| HEMA | 8.85 (92.93) * | 1.93 (0.62) * | 4.80 (6.22) ** | |||||
| MeHQ | ||||||||
| MOPA | 160.60 (27.61) | 149.38 (181.21) | ||||||
| TMPTMA | 0.40 (0.15) | |||||||
| TPSb | ||||||||
| Activa Bioactive Restorative | Ena HRi | Enamel Plus Hri BioFunction | GC Fuji II LC | |||||
|---|---|---|---|---|---|---|---|---|
| 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | 24 h | 1 m | |
| MS | 43.77 (20.25) | 81.28 (27.59) ** | ||||||
| ACP | 59.71 (23.96) | 95.95 (34.29) | ||||||
| MCE | 110.00 (49.18) | 164.00 (81.43) | ||||||
| BHT | 3.91 (3.38) * | 12.58 (7.05) *,** | 11.58 (5.46) * | 29.60 (58.57) *,** | ||||
| BPA | 0.91 (0.74) | 0.72 (0.76) | ||||||
| 1,4-BDDMA | 554.23 (217.34) * | 369.27 (121.63) * | 603.47 (295.91) * | 636.15 (311.61) * | 137.43 (107.92) * | 104.34 (158.85) * | ||
| CQ | 12.08 (2.02) * | 28.19 (8.26) *,** | 5.20 (2.75) * | 8.70 (5.34) * | 1.19 (0.97) * | 3.16 (4.79) *,** | 10.85 (1.83) * | 24.22 (14.47) *,** |
| DMABEE | 33.97 (19.55) * | 99.02 (38.73) *,** | 5.74 (4.87) * | 13.90 (15.13) *,** | 4.62 (3.69) * | 18.63 (10.72) *,** | ||
| DBP | ||||||||
| HEMA | 694.44 (229.71) * | 854.14 (158.63) * | 306.72 (235.00) * | 363.76 (237.71) * | 178.95 (116.71) * | 169.28 (141.01) * | 25.76 (9.16) * | 20.75 (4.69) * |
| MeHQ | 7.18 (8.17) | |||||||
| MOPA | 890.78 (576.86) | 1372.61 (430.58) | ||||||
| TMPTMA | 40.11 (24.31) | 64.40 (21.33) | ||||||
| TPSb | 0.47 (0.69) | 4.39 (1.88) ** | ||||||
| Si | Al | Ca | Na | Ba | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 w | 2 w | 1 w | 2 w | 1 w | 2 w | 1 w | 2 w | 1 w | 2 w | |
| Activa Bioactive Restorative | 1.22 ± 0.29 | 0 *,** | 1.08 ± 0.68 | 0.46 ± 0.03 *,** | 0.7 ± 0.21 ** | 0.69 ± 0.08 | 2.71 ± 0.14 | 2.21 ± 0.04 | 0.16 ± 0.03 | 0.06 ± 0.02 |
| Ena HRi | 0.8 ± 0.2 | 0 *,** | 0.87 ± 0.87 | 0.85 ± 0.17 * | 0.19 ± 0.05 | 0.6 ± 0.2 ** | 2.38 ± 0.34 | 2.09 ± 0.02 | 0.97 ± 0.02 *,** | 0.06 ± 0.008 ** |
| Enamel Plus HRi Biofunction | 0.27 ± 0.08 * | 0 * | 0.31 ± 0.06 * | 0.81 ± 0.54 *,** | 0.29 ± 0.02 | 0.78 ± 0.09 ** | 2.2 ± 0.11 | 0 ** | 0.07 ± 0.01 | 0.06 ± 0.01 |
| Fuji II LC | 0.91 ± 0.59 | 0.41 ± 0.3 *,** | 2.34 ± 0.3 * | 0.84 ± 0.44 *,** | 0.75 ± 0.29 ** | 0.39 ± 0.42 *,** | 3.65 ± 0.26 | 2.39 ± 0.12 | 0.11 ± 0.01 | 0.09 ± 0.01 |
| Fuji IX | 11.17 ± 2.12 *,** | 2.46 ± 0.8 *,** | 5.73 ± 0.81 *,** | 1.18 ± 0.24 *,** | 0.25 ± 0.05 | 0.7 ± 0.39 ** | 7.32 ± 0.74 *,** | 3.22 ± 0.2 ** | 0.11 ± 0.03 | 0.09 ± 0.008 |
| 24 h Extract/24 h Measurement | ||||
|---|---|---|---|---|
| 100% | 75% | 50% | 25% | |
| Ena HRi | 101 ± 9.54 | 102 ± 6.56 | 97 ± 3.46 | 101.33 ± 2.89 |
| Activa Bioactive Restorative | 109 ± 5.13 | 108 ± 3.61 | 102 ± 4 | 101.7 ± 2.08 |
| 24 h extract/72 h measurement | ||||
| 100% | 75% | 50% | 25% | |
| Ena HRi | 97 ± 13 | 96.7 ± 9.02 | 95 ± 4.51 | 99 ± 3 |
| Activa Bioactive Restorative | 116 ± 13.87 | 113 ± 11.93 | 108 ± 6.65 | 105 ± 4.73 |
| 1 week extract/24 h measurement | ||||
| 100% | 75% | 50% | 25% | |
| Ena HRi | 115 ± 30.75 | 113 ± 25.24 | 108 ± 20.22 | 111 ± 22.81 |
| Activa Bioactive Restorative | 108 ± 7.81 | 108 ± 7.5 | 116 ± 15.8 | 107 ± 9.64 |
| 1 week extract/72 h measurement | ||||
| 100% | 75% | 50% | 25% | |
| Ena HRi | 75 ± 26.1 * | 75 ± 23.46 * | 83 ± 16.01 | 89.7 ± 34.67 |
| Activa Bioactive Restorative | 99 ± 10.58 | 101 ± 6.65 | 98 ± 8.5 | 99.7 ± 3.79 |
| Material | Manufacturer | Composition according to MSDS |
|---|---|---|
| Activa Bioactive Restorative | Pulpdent, Watertown, MA, USA | Blend of diurethane and other methacrylates with modified polyacrylic acid (44.6%) Silica, amorphous (6.7%) Sodium fluoride (0.75%) |
| Ena HRi | Micerium S.p.A., Avegno, Italy | 1,4-Butanediol dimethacrylate Urethane dimethacrylate Bis-GMA Tetramethylenedimethacrylate (2.5–10%) * CONTENT OF THE FILLERS: 74% by weight (60% by volume); particle size of highly dispersed silicone dioxide is 0.005–0.05 μm, glass fillers have a particle size of 0.2–3.0 μm. |
| Enamel Plus HRi BioFunction | Micerium S.p.A., Avegno, Italy | Tricyclodecanedimethanoldimethacrylate (10–25%) 2-propenoic acid, 2-methyl-, 3- (trimethoxysilyl) propyl ester, reaction products with Silicon dioxide (2.5–10%) * TOTAL CONTENT OF THE FILLERS: 74% by weight (60% by volume); particle size of highly dispersed silicone dioxide is 0.005–0.05 μm, glass fillers have a particle size of 0.2–3.0 μm |
| GC Fuji II LC | GC America Inc., Alsip, IL, USA | 2-hydroxyethyl methacrylate (HEMA) (25–50%) Polybasic carboxylic acid (5–10%) Urethane dimethacrylate (UDMA) (1–5%) Dimethacrylate (1–5%) |
| GC Fuji IX | GC America Inc., Alsip, IL, USA | Polybasic carboxylic acid (5–10%) |
| Ion | Wavelength (nm) |
|---|---|
| Si | 251.611 |
| Al | 396.153 |
| Ca | 393.366 |
| P | 213.617 |
| Na Ba | 588.995 455.403 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roussou, K.; Nikolaidis, A.K.; Ziouti, F.; Arhakis, A.; Arapostathis, K.; Koulaouzidou, E.A. Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials. Molecules 2021, 26, 4912. https://doi.org/10.3390/molecules26164912
Roussou K, Nikolaidis AK, Ziouti F, Arhakis A, Arapostathis K, Koulaouzidou EA. Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials. Molecules. 2021; 26(16):4912. https://doi.org/10.3390/molecules26164912
Chicago/Turabian StyleRoussou, Konstantina, Alexandros K. Nikolaidis, Fani Ziouti, Aristidis Arhakis, Konstantinos Arapostathis, and Elisabeth A. Koulaouzidou. 2021. "Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials" Molecules 26, no. 16: 4912. https://doi.org/10.3390/molecules26164912
APA StyleRoussou, K., Nikolaidis, A. K., Ziouti, F., Arhakis, A., Arapostathis, K., & Koulaouzidou, E. A. (2021). Cytotoxic Evaluation and Determination of Organic and Inorganic Eluates from Restorative Materials. Molecules, 26(16), 4912. https://doi.org/10.3390/molecules26164912