RETRACTED: Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives
Abstract
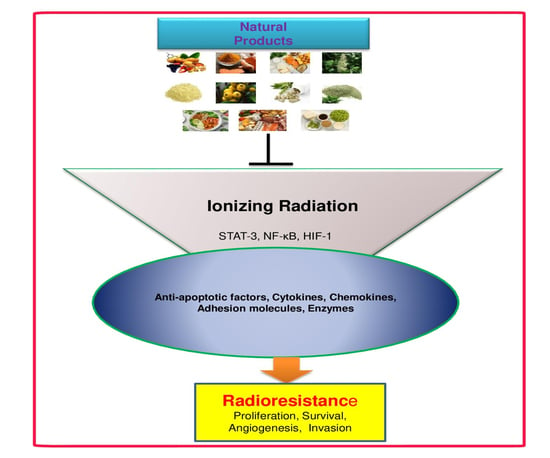
:1. Introduction
2. Resveratrol
3. Curcumin
4. Vitamin D
5. Celastrol
6. Zerumbone
7. Ursolic Acid
8. Withaferin A
9. Emodin
10. Berberine
11. Selenium
12. Genistein
13. Future Perspectives
14. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviation
| ACF | Aberrant crypt foci |
| AGEs | Advanced glycation end products |
| Akt | Protein kinase B |
| AP-1 | Activator protein 1 |
| ATM | Ataxia telangiectasia mutated |
| DNA | Deoxyribonucleic acid |
| EGFR | Epidermal growth factor receptor |
| Erg-1 | Early growth response protein 1 |
| FoxO1 | Forkhead box protein O1 |
| GBM | Glioblastoma multiform |
| HIF-1 | Hypoxia-inducible factor 1 |
| HMEC | Human mammary epithelial cells |
| IKKα | IκB kinase α |
| IR | Ionizing radiation |
| JAK2 | Janus kinase 2 |
| MAPK | Mitogen activated protein kinase |
| NF-κB | Nuclear factor kappa B |
| NK | Natural killer cells |
| Nrf2 | Nuclear factor erythroid 2-related factor 2 |
| NSCLC | Non-small cell lung cancer |
| PD | Pharmacodynamic |
| PI3K | Phosphoinositide 3-kinase |
| PK | Pharmacokinetic |
| PPAR- α | Peroxisome proliferator-activated receptor alpha |
| ROS | Reactive oxygen species |
| RT | Radiotherapy |
| RV | Resveratrol |
| STAT3 | Transducer and activator of transcription 3 |
| TBK1 | TANK-binding kinase 1 |
| UA | Ursolic acid |
| VDR | Vitamin D receptor |
| VEGF | Vascular endothelial growth factor |
| WA | Withaferin A |
| WBC | White blood cells |
| ZER | Zingiber |
References
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Pharmacotherapy, Multiple strategies with the synergistic approach for addressing colorectal cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef]
- Epstein, J.; Sanderson, I.R.; MacDonald, T.T. Curcumin as a therapeutic agent: The evidence from in vitro, animal and human studies. Br. J. Nutr. 2010, 103, 1545–1557. [Google Scholar] [CrossRef]
- Forte, G.I.; Minafra, L.; Bravatà, V.; Cammarata, F.P.; Lamia, D.; Pisciotta, P.; Cirrone, G.A.P.; Cuttone, G.; Gilardi, M.C.; Russo, G. Radiogenomics: The utility in patient selection. Transl. Cancer Res. 2017, 6, S852–S874. [Google Scholar] [CrossRef]
- Bravatà, V.; Cava, C.; Minafra, L.; Cammarata, F.P.; Russo, G.; Gilardi, M.C.; Castiglioni, I.; Forte, G.I. Radiation-induced gene expression changes in high and low grade breast cancer cell types. Int. J. Mol. Sci. 2018, 19, 1084. [Google Scholar] [CrossRef]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.-W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Med. Sci. 2012, 9, 193. [Google Scholar] [CrossRef]
- Calvaruso, M.; Pucci, G.; Musso, R.; Bravatà, V.; Cammarata, F.P.; Russo, G.; Forte, G.I.; Minafra, L. Nutraceutical Compounds as Sensitizers for Cancer Treatment in Radiation Therapy. Int. J. Mol. Sci. 2019, 20, 5267. [Google Scholar] [CrossRef] [PubMed]
- Grimes, D.R.; Partridge, M. A mechanistic investigation of the oxygen fixation hypothesis and oxygen enhancement ratio. Biomed. Phys. Eng. Express. 2015, 1, 045209. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.; Hong, C.R.; Wong, W.W.; Liew, L.P.; Shome, A.; Wang, J.; Gu, Y.; Stevenson, R.J.; Qi, W.; Anderson, R.F. Next-generation hypoxic cell radiosensitizers: Nitroimidazole alkylsulfonamides. J. Med. Chem. 2018, 61, 1241–1254. [Google Scholar] [CrossRef] [PubMed]
- Rauth, A. Pharmacology and toxicology of sensitizers: Mechanism studies. Int. J. Radiat. Oncol. Biol. Phys. 1984, 10, 1293–1300. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Wang, F.-X.; Jia, K.-K.; Kong, L.-D.J.F. Natural product interventions for chemotherapy and radiotherapy-induced side effects. Front. Pharmacol. 2018, 9, 1253. [Google Scholar] [CrossRef]
- Tagde, P.; Tagde, P.; Tagde, S.; Bhattacharya, T.; Garg, V.; Akter, R.; Rahman, M.H.; Najda, A.; Albadrani, G.M.; Sayed, A.A.J. Natural bioactive molecules: An alternative approach to the treatment and control of glioblastoma multiforme. Biomed. Pharmacother. 2021, 141, 111928. [Google Scholar] [CrossRef]
- Youn, H.S.; Lee, J.Y.; Fitzgerald, K.A.; Young, H.A.; Akira, S.; Hwang, D.H. Specific inhibition of MyD88-independent signaling pathways of TLR3 and TLR4 by resveratrol: Molecular targets are TBK1 and RIP1 in TRIF complex. J. Immunol. 2005, 175, 3339–3346. [Google Scholar] [CrossRef]
- Signorelli, P.; Ghidoni, R. Resveratrol as an anticancer nutrient: Molecular basis, open questions and promises. J. Nutr. Biochem. 2005, 16, 449–466. [Google Scholar] [CrossRef]
- Shankar, S.; Singh, G.; Srivastava, R.K. Chemoprevention by resveratrol: Molecular mechanisms and therapeutic potential. Front. Biosci. 2007, 12, 4839–4854. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Behl, T.; Chowdhury, M.A.; Mohammed, M.; Bulbul, I.J.; Elshenawy, S.E.; Kamal, M.A. COVID-19 outbreak and emerging management through pharmaceutical therapeutic strategy. Curr. Pharm. Des. 2020, 26, 5224–5240. [Google Scholar] [CrossRef] [PubMed]
- Renaud, S.D.; de Lorgeril, M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet 1992, 339, 1523–1526. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhang, C.-X.; Liu, Y.-M.; Chen, K.-L.; Chen, G. A comparative study of anti-aging properties and mechanism: Resveratrol and caloric restriction. Oncotarget 2017, 8, 65717. [Google Scholar] [CrossRef]
- Rahman, M.H.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.J. Resveratrol and Neuroprotection: Impact and its Therapeutic Potential in Alzheimer’s disease. Front. Pharmacol. 2020, 11, 619024. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Rahman, M.; Hossain, M.; Biswas, P.; Islam, R.; Uddin, M.J.; Rhim, H.J. Molecular insights into the multifunctional role of natural compounds: Autophagy modulation and cancer prevention. Biomedicines 2020, 8, 517. [Google Scholar] [CrossRef]
- Luo, H.; Yang, A.; Schulte, B.A.; Wargovich, M.J.; Wang, G.Y. Resveratrol induces premature senescence in lung cancer cells via ROS-mediated DNA damage. PLoS ONE 2013, 8, e60065. [Google Scholar] [CrossRef]
- Chen, Y.-A.; Lien, H.-M.; Kao, M.-C.; Lo, U.-G.; Lin, L.-C.; Lin, C.-J.; Chang, S.-J.; Chen, C.-C.; Hsieh, J.-T.; Lin, H. Sensitization of radioresistant prostate cancer cells by resveratrol isolated from arachis hypogaea stems. PLoS ONE 2017, 12, e0169204. [Google Scholar] [CrossRef]
- Tan, Y.; Wei, X.; Zhang, W.; Wang, X.; Wang, K.; Du, B.; Xiao, J. Resveratrol enhances the radiosensitivity of nasopharyngeal carcinoma cells by downregulating E2F1. Oncol. Rep. 2017, 37, 1833–1841. [Google Scholar] [CrossRef]
- da Costa Araldi, I.C.; Bordin, F.P.R.; Cadoná, F.C.; Barbisan, F.; Azzolin, V.F.; Teixeira, C.F.; Baumhardt, T.; da Cruz, I.B.M.; Duarte, M.M.M.F.; de Freitas Bauermann, L. The in vitro radiosensitizer potential of resveratrol on MCF-7 breast cancer cells. Chem. Biol. Interact. 2018, 282, 85–92. [Google Scholar] [CrossRef]
- Tomé-Carneiro, J.; Gonzálvez, M.; Larrosa, M.; Yáñez-Gascón, M.J.; García-Almagro, F.J.; Ruiz-Ros, J.A.; Tomás-Barberán, F.A.; García-Conesa, M.T.; Espín, J.C. Grape resveratrol increases serum adiponectin and downregulates inflammatory genes in peripheral blood mononuclear cells: A triple-blind, placebo-controlled, one-year clinical trial in patients with stable coronary artery disease. Cardiovasc. Drugs. Ther. 2013, 27, 37–48. [Google Scholar] [CrossRef]
- Anthwal, A.; Thakur, B.K.; Rawat, M.; Rawat, D.; Tyagi, A.K.; Aggarwal, B.B. Synthesis, characterization and in vitro anticancer activity of C-5 curcumin analogues with potential to inhibit TNF-α-induced NF-κB activation. BioMed Res. Int. 2014, 2014, 524161. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Mano, V.; Radhakrishnan, A.; Janani, S.; Akter, R.; Kaushik, D.; Rahman, M.H. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp. Gerontol. 2021, 152, 111438. [Google Scholar] [CrossRef]
- Abdulghani, J.; Gu, L.; Dagvadorj, A.; Lutz, J.; Leiby, B.; Bonuccelli, G.; Lisanti, M.P.; Zellweger, T.; Alanen, K.; Mirtti, T.J.T.A. Stat3 promotes metastatic progression of prostate cancer. Am. J. Pathol. 2008, 172, 1717–1728. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lee, Y.-M.; Chang, G.-C.; Yu, S.-L.; Hsieh, W.-Y.; Chen, J.J.; Chen, H.-W.; Yang, P.-C.J.P. Curcumin induces EGFR degradation in lung adenocarcinoma and modulates p38 activation in intestine: The versatile adjuvant for gefitinib therapy. PLoS ONE 2011, 6, e23756. [Google Scholar] [CrossRef] [PubMed]
- Kabir, M.; Rahman, M.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M. Potential Role of Curcumin and Its Nanoformulations to Treat Various Types of Cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Davie, J.R.; He, S.; Li, L.; Sekhavat, A.; Espino, P.; Drobic, B.; Dunn, K.L.; Sun, J.-M.; Chen, H.Y.; Yu, J.J.A. Nuclear organization and chromatin dynamics–Sp1, Sp3 and histone deacetylases. Adv. Enzym. Regul. 2008, 48, 189–208. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rahman, H.; Behl, T.; Chowdhury, M.; Rahman, A.; Manirujjaman, M.; Bulbul, I.J.; Elshenaw, S.E.; Tit, D.M.; Bungau, S. Prospective role of polyphenolic compounds in the treatment of neurodegenerative diseases. CNS Neurol. Disord. Drug Targets. 2020. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, T.; Dutta, S.; Akter, R.; Rahman, M.; Karthika, C.; Nagaswarupa, H.P.; Murthy, H.C.A.; Fratila, O.; Brata, R.; Bungau, S. Role of Phytonutrients in Nutrigenetics and Nutrigenomic Perspective in Curing Breast Cancer. Biomolecules 2021, 11, 1176. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Koh, W.; Aggarwal, B.B. Discovery of curcumin, a component of golden spice, and its miraculous biological activities. Clin. Exp. Pharmacol. Physiol. 2012, 39, 283–299. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.A.; Euden, S.A.; Platton, S.L.; Cooke, D.N.; Shafayat, A.; Hewitt, H.R.; Marczylo, T.H.; Morgan, B.; Hemingway, D.; Plummer, S.M. Phase I clinical trial of oral curcumin: Biomarkers of systemic activity and compliance. Clin. Cancer Res. 2004, 10, 6847–6854. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Hsieh, C. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Sharma, R.A.; McLelland, H.R.; Hill, K.A.; Ireson, C.R.; Euden, S.A.; Manson, M.M.; Pirmohamed, M.; Marnett, L.J.; Gescher, A.J.; Steward, W.P. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin. Cancer Res. 2001, 7, 1894–1900. [Google Scholar]
- Asai, A.; Miyazawa, T. Occurrence of orally administered curcuminoid as glucuronide and glucuronide/sulfate conjugates in rat plasma. Life Sci. 2000, 67, 2785–2793. [Google Scholar] [CrossRef]
- European Union (EU) Council Directive 91/676/EEC of 12 December 1991 Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources. Eur. Union Bruss. Belg. 1991. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31991L0676 (accessed on 22 September 2021).
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Carroll, R.E.; Benya, R.V.; Turgeon, D.K.; Vareed, S.; Neuman, M.; Rodriguez, L.; Kakarala, M.; Carpenter, P.M.; McLaren, C.; Meyskens, F.L. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev. Res. 2011, 4, 354–364. [Google Scholar] [CrossRef]
- Kanai, M.; Yoshimura, K.; Asada, M.; Imaizumi, A.; Suzuki, C.; Matsumoto, S.; Nishimura, T.; Mori, Y.; Masui, T.; Kawaguchi, Y. A phase I/II study of gemcitabine-based chemotherapy plus curcumin for patients with gemcitabine-resistant pancreatic cancer. Cancer Chemother. Pharmacol. 2011, 68, 157–164. [Google Scholar] [CrossRef]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Antunes, L.M.G.; Araújo, M.C.P.; Darin, J.D.A.C.; Maria de Lourdes, P.B.J.M.R.G.T.; Mutagenesis, E. Effects of the antioxidants curcumin and vitamin C on cisplatin-induced clastogenesis in Wistar rat bone marrow cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2000, 465, 131–137. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, X.-L.; He, D.-H.; Cheng, Y.-X. Protection against chemotherapy-and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2020, 80, 153402. [Google Scholar] [CrossRef]
- Holick, M.F. Vitamin D: Its role in cancer prevention and treatment. Prog. Biophys. Mol. Biol. 2006, 92, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Lazarus, H.; Haynesworth, S.; Gerson, S.; Rosenthal, N.; Caplan, A. Ex vivo expansion and subsequent infusion of human bone marrow-derived stromal progenitor cells (mesenchymal progenitor cells): Implications for therapeutic use. Bone Marrow Transplant. 1995, 16, 557–564. [Google Scholar]
- Rosen, C.J. Vitamin D insufficiency. N. Engl. J. Med. 2011, 364, 248–254. [Google Scholar] [CrossRef]
- Plum, L.A.; DeLuca, H.F. The functional metabolism and molecular biology of vitamin D action. Clin. Cases. Miner. Bone Metab. 2009, 7, 20–41. [Google Scholar] [CrossRef]
- Norman, A.W.; Bouillon, R. Vitamin D nutritional policy needs a vision for the future. Exp. Biol. Med. 2010, 235, 1034–1045. [Google Scholar] [CrossRef]
- Feldman, D.; Krishnan, A.V.; Swami, S.; Giovannucci, E.; Feldman, B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer 2014, 14, 342–357. [Google Scholar] [CrossRef]
- Ingraham, B.A.; Bragdon, B.; Nohe, A. Molecular basis of the potential of vitamin D to prevent cancer. Curr. Med. Res. Opin. 2008, 24, 139–149. [Google Scholar] [CrossRef]
- Masuda, S.; Jones, G. Promise of vitamin D analogues in the treatment of hyperproliferative conditions. Mol. Cancer Ther. 2006, 5, 797–808. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, R.; Carmeliet, G.; Verlinden, L.; van Etten, E.; Verstuyf, A.; Luderer, H.F.; Lieben, L.; Mathieu, C.; Demay, M. Vitamin D and human health: Lessons from vitamin D receptor null mice. Endocr. Rev. 2008, 29, 726–776. [Google Scholar] [CrossRef] [PubMed]
- Cihan, Y.B. Does vitamin D prevent radiotherapy-induced toxicity? Turkish J. Biochem. 2019, 44, 575–577. [Google Scholar] [CrossRef]
- Parrón, T.; Requena, M.; Hernández, A.F.; Alarcón, R. Environmental exposure to pesticides and cancer risk in multiple human organ systems. Toxicol. Lett. 2014, 230, 157–165. [Google Scholar] [CrossRef]
- Cui, Y.; Rohan, T.E. Vitamin D, calcium, and breast cancer risk: A review. Cancer Epidemiol. Biomark. Prev. 2006, 15, 1427–1437. [Google Scholar] [CrossRef] [PubMed]
- John, E.M.; Koo, J.; Schwartz, G.G. Sun exposure and prostate cancer risk: Evidence for a protective effect of early-life exposure. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1283–1286. [Google Scholar] [CrossRef] [PubMed]
- Gupta, D.; Lammersfeld, C.; Trukova, K.; Lis, C. Vitamin D and prostate cancer risk: A review of the epidemiological literature. Prostate Cancer Prostatic Dis. 2009, 12, 215–226. [Google Scholar] [CrossRef]
- Waltz, P.; Chodick, G. Assessment of ecological regression in the study of colon, breast, ovary, non-Hodgkin’s lymphoma, or prostate cancer and residential UV. Eur. J. Cancer Prev. 2008, 17, 279–286. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, Y.-F. Natural compounds as anticancer agents: Experimental evidence. World J. Exp. Med. 2012, 2, 45. [Google Scholar] [CrossRef]
- Hathcock, J.N.; Shao, A.; Vieth, R.; Heaney, R. Risk assessment for vitamin D. Am. J. Clin. Nutr. 2007, 85, 6–18. [Google Scholar] [CrossRef]
- Jones, G. Pharmacokinetics of vitamin D toxicity. Am. J. Clin. Nutr. 2008, 88, S582–S586. [Google Scholar] [CrossRef]
- Ma, Y.; Khalifa, B.; Yee, Y.K.; Lu, J.; Memezawa, A.; Savkur, R.S.; Yamamoto, Y.; Chintalacharuvu, S.R.; Yamaoka, K.; Stayrook, K.R. Identification and characterization of noncalcemic, tissue-selective, nonsecosteroidal vitamin D receptor modulators. J. Clin. Investig. 2006, 116, 892–904. [Google Scholar] [CrossRef] [PubMed]
- Beer, T.M.; Lemmon, D.; Lowe, B.A.; Henner, W.D. High-dose weekly oral calcitriol in patients with a rising PSA after prostatectomy or radiation for prostate carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1217–1224. [Google Scholar] [CrossRef]
- Trump, D.L.; Potter, D.M.; Muindi, J.; Brufsky, A.; Johnson, C.S. Phase II trial of high-dose, intermittent calcitriol (1, 25 dihydroxyvitamin D3) and dexamethasone in androgen-independent prostate cancer. Cancer 2006, 106, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Bufu, T.; Di, X.; Yilin, Z.; Gege, L.; Xi, C.; Ling, W.J. Celastrol inhibits colorectal cancer cell proliferation and migration through suppression of MMP3 and MMP7 by the PI3K/AKT signaling pathway. Anticancer Drugs 2018, 29, 530–538. [Google Scholar] [CrossRef]
- Wang, G.; Fersht, A.R. Multisite aggregation of p53 and implications for drug rescue. Proc. Natl. Acad. Sci. USA 2017, 114, E2634–E2643. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, Y.; Fan, Y.; Zhou, D.J. Celastrol inhibits the growth of human glioma xenografts in nude mice through suppressing VEGFR expression. Cancer Lett. 2008, 264, 101–106. [Google Scholar] [CrossRef]
- Lee, J.-H.; Choi, K.J.; Seo, W.D.; Jang, S.Y.; Kim, M.; Lee, B.W.; Kim, J.Y.; Kang, S.; Park, K.H.; Lee, Y.-S. Enhancement of radiation sensitivity in lung cancer cells by celastrol is mediated by inhibition of Hsp90. Int. J. Mol. Med. 2011, 27, 441–446. [Google Scholar] [PubMed]
- Seo, H.R.; Seo, W.D.; Pyun, B.-J.; Lee, B.W.; Jin, Y.B.; Park, K.H.; Seo, E.-K.; Lee, Y.-J.; Lee, Y.-S. Radiosensitization by celastrol is mediated by modification of antioxidant thiol molecules. Chem. Biol. Interact. 2011, 193, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.Y.; Kim, T.-H.; Choi, J.W.; Lee, Y.H.; Lee, K.K.; Yoon, K.-H. Evaluation of connectivity map-discovered celastrol as a radiosensitizing agent in a murine lung carcinoma model: Feasibility study of diffusion-weighted magnetic resonance imaging. PLoS ONE 2017, 12, e0178204. [Google Scholar] [CrossRef] [PubMed]
- Fusi, F.; Durante, M.; Sgaragli, G.; Khanh, P.N.; Huong, T.T.; Cuong, N.M. In vitro vasoactivity of zerumbone from Zingiber zerumbet. Planta Med. 2015, 81, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Yob, N.; Jofrry, S.M.; Affandi, M.; Teh, L.; Salleh, M.; Zakaria, Z. Zingiber zerumbet (L.): A review of its ethnomedicinal, chemical, and pharmacological uses. Evid. Based Complementary Altern. Med. 2011, 2011, 543216. [Google Scholar] [CrossRef] [PubMed]
- Rahman, H.S.; Rasedee, A.; Yeap, S.K.; Othman, H.H.; Chartrand, M.S.; Namvar, F.; Abdul, A.B.; How, C.W. Biomedical properties of a natural dietary plant metabolite, zerumbone, in cancer therapy and chemoprevention trials. BioMed Res. Int. 2014, 2014, 920742. [Google Scholar] [CrossRef]
- Murakami, A.; Tanaka, T.; Lee, J.Y.; Surh, Y.J.; Kim, H.W.; Kawabata, K.; Nakamura, Y.; Jiwajinda, S.; Ohigashi, H. Zerumbone, a sesquiterpene in subtropical ginger, suppresses skin tumor initiation and promotion stages in ICR mice. Int. J. Cancer 2004, 110, 481–490. [Google Scholar] [CrossRef]
- Sung, B.; Jhurani, S.; Ahn, K.S.; Mastuo, Y.; Yi, T.; Guha, S.; Liu, M.; Aggarwal, B.B. Zerumbone down-regulates chemokine receptor CXCR4 expression leading to inhibition of CXCL12-induced invasion of breast and pancreatic tumor cells. Cancer Res. 2008, 68, 8938–8944. [Google Scholar] [CrossRef]
- Choi, S.-H.; Lee, Y.-J.; Seo, W.D.; Lee, H.-J.; Nam, J.-W.; Lee, Y.J.; Kim, J.; Seo, E.-K.; Lee, Y.-S. Altered cross-linking of HSP27 by zerumbone as a novel strategy for overcoming HSP27-mediated radioresistance. Int. J. Radiat. Oncol. Biol. Phys. 2011, 79, 1196–1205. [Google Scholar] [CrossRef]
- Huang, G.-C.; Chien, T.-Y.; Chen, L.-G.; Wang, C.-C. Antitumor effects of zerumbone from Zingiber zerumbet in P-388D1 cells in vitro and in vivo. Planta Med. 2005, 71, 219–224. [Google Scholar] [CrossRef]
- Chiang, P.-K.; Tsai, W.-K.; Chen, M.; Lin, W.-R.; Chow, Y.-C.; Lee, C.-C.; Hsu, J.-M.; Chen, Y.-J. Zerumbone regulates DNA repair responding to Ionizing radiation and enhances radiosensitivity of human prostatic cancer cells. Integr. Cancer Ther. 2018, 17, 292–298. [Google Scholar] [CrossRef]
- Bousselham, A.; Bouattane, O.; Youssfi, M.; Raihani, A.J. Brain tumor temperature effect extraction from MRI imaging using bioheat equation. Procedia Comput. Sci. 2018, 127, 336–343. [Google Scholar] [CrossRef]
- Kansal, A.R.; Torquato, S.; Harsh, G.; Chiocca, E.; Deisboeck, T.J. Simulated brain tumor growth dynamics using a three-dimensional cellular automaton. J. Theor. Biol. 2000, 203, 367–382. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.-Y.; Hsu, M.-J.; Wang, C.-C.; Chen, B.-C.; Hong, C.-Y.; Chen, M.-C.; Chiu, W.-T.; Lin, C.-H. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J. Biomed. Sci. 2012, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Woźniak, Ł.; Skąpska, S.; Marszałek, K. Ursolic acid—A pentacyclic triterpenoid with a wide spectrum of pharmacological activities. Molecules 2015, 20, 20614–20641. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.J.; Tak, J.K.; Kim, S.T.; Nam, W.S.; Kim, S.Y.; Park, K.M.; Park, J.-W. Sensitization of ionizing radiation-induced apoptosis by ursolic acid. Free Radic. Res. 2012, 46, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-H.; Wang, E.; Kumar, N.; Glickman, R.D. Ursolic acid differentially modulates apoptosis in skin melanoma and retinal pigment epithelial cells exposed to UV–VIS broadband radiation. Apoptosis 2014, 19, 816–828. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Zhang, Q.; Yu, M.; Qi, X.; Wang, G.; Xiao, L.; Yi, Q.; Jin, W. Ursolic acid sensitizes radioresistant NSCLC cells expressing HIF-1α through reducing endogenous GSH and inhibiting HIF-1α. Oncol. Lett. 2017, 13, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.M.; Lu, J.; Zhang, Y.Q.; Zheng, Y.L.; Hu, B.; Cheng, W.; Zhang, Z.-F.; Li, M.Q. Ursolic acid improves domoic acid-induced cognitive deficits in mice. Toxicol. Appl. Pharmacol. 2013, 271, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Wu, D.-M.; Zheng, Y.-L.; Hu, B.; Zhang, Z.-F.; Ye, Q.; Liu, C.-M.; Shan, Q.; Wang, Y.-J. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-κB pathway activation. Cereb. Cortex. 2010, 20, 2540–2548. [Google Scholar] [CrossRef] [PubMed]
- Kupchan, S.M.; Doskotch, R.W.; Bollinger, P.; McPhail, A.; Sim, G.; Renauld, J.S. The Isolation and Structural Elucidation of a Novel Steroidal Tumor Inhibitor from Acnistus arborescens1, 2. J. Am. Chem. Soc. 1965, 87, 5805–5806. [Google Scholar] [CrossRef] [PubMed]
- Shohat, B.; Gitter, S.; Abraham, A.; Lavie, D. Antitumor activity of withaferin A (NSC-101088). Cancer Chemother. Rep. 1967, 51, 271. [Google Scholar] [PubMed]
- Lee, I.-C.; Choi, B.Y. Withaferin-A—A natural anticancer agent with pleitropic mechanisms of action. Int. J. Mol. Sci. 2016, 17, 290. [Google Scholar] [CrossRef]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U.; Udupa, N.; Srinivasan, K.K. Antitumor and radiosensitizing effects of withaferin A on mouse Ehrlich ascites carcinoma in vivo. Acta Oncol. 1996, 35, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Kamath, R.; Rao, B.S.; Devi, P.U. Response of a mouse fibrosarcoma to withaferin A and radiation. Pharm. Pharmacol. Commun. 1999, 5, 287–291. [Google Scholar] [CrossRef]
- Murata, R.; Siemann, D.W.; Overgaard, J.; Horsman, M.R. Improved tumor response by combining radiation and the vascular-damaging drug 5, 6-dimethylxanthenone-4-acetic acid. Radiat. Res. 2001, 156, 503–509. [Google Scholar] [CrossRef]
- Oh, J.H.; Lee, T.-J.; Kim, S.H.; Choi, Y.H.; Lee, S.H.; Lee, J.M.; Kim, Y.-H.; Park, J.-W.; Kwon, T.K. Induction of apoptosis by withaferin A in human leukemia U937 cells through down-regulation of Akt phosphorylation. Apoptosis 2008, 13, 1494–1504. [Google Scholar] [CrossRef]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Combination of withaferin A and X-ray irradiation enhances apoptosis in U937 cells. Toxicol. Vitr. 2011, 25, 1803–1810. [Google Scholar] [CrossRef]
- Yang, E.S.; Choi, M.J.; Kim, J.H.; Choi, K.S.; Kwon, T.K. Withaferin A enhances radiation-induced apoptosis in Caki cells through induction of reactive oxygen species, Bcl-2 downregulation and Akt inhibition. Chem. Biol. Interact. 2011, 190, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Widodo, N.; Kaur, K.; Shrestha, B.G.; Takagi, Y.; Ishii, T.; Wadhwa, R.; Kaul, S.C. Selective killing of cancer cells by leaf extract of Ashwagandha: Identification of a tumor-inhibitory factor and the first molecular insights to its effect. Clin. Cancer Res. 2007, 13, 2298–2306. [Google Scholar] [CrossRef] [PubMed]
- Hahm, E.-R.; Moura, M.B.; Kelley, E.E.; Van Houten, B.; Shiva, S.; Singh, S.V. Withaferin A-induced apoptosis in human breast cancer cells is mediated by reactive oxygen species. PLoS ONE 2011, 6, e23354. [Google Scholar] [CrossRef]
- Sehrawat, A.; Samanta, S.K.; Hahm, E.-R.; Croix, C.S.; Watkins, S.; Singh, S.V. Withaferin A-mediated apoptosis in breast cancer cells is associated with alterations in mitochondrial dynamics. Mitochondrion 2019, 47, 282–293. [Google Scholar] [CrossRef]
- Li, L.; Tian, Y.; Yu, J.; Song, X.; Jia, R.; Cui, Q.; Tong, W.; Zou, Y.; Li, L.; Yin, L. iTRAQ-based quantitative proteomic analysis reveals multiple effects of Emodin to Haemophilus parasuis. J. Proteomics 2017, 166, 39–47. [Google Scholar] [CrossRef]
- Chen, T.; Zheng, L.Y.; Xiao, W.; Gui, D.; Wang, X.; Wang, N. Emodin ameliorates high glucose induced-podocyte epithelial-mesenchymal transition in-vitro and in-vivo. Cell. Physiol. Biochem. 2015, 35, 1425–1436. [Google Scholar] [CrossRef]
- Liu, Y.; Hou, H.; Li, D.; Cheng, D.; Qin, C.; Li, W. Enhancement effect of emodin on radiosensitivity of human nasopharyngeal carcinoma transplanted in nude mice. Chin. Pharm. J. 2010, 45, 1331–1334. [Google Scholar]
- Shrimali, D.; Shanmugam, M.K.; Kumar, A.P.; Zhang, J.; Tan, B.K.; Ahn, K.S.; Sethi, G. Targeted abrogation of diverse signal transduction cascades by emodin for the treatment of inflammatory disorders and cancer. Cancer Lett. 2013, 341, 139–149. [Google Scholar] [CrossRef]
- Dey, D.; Ray, R.; Hazra, B. Antitubercular and antibacterial activity of quinonoid natural products against multi-drug resistant clinical isolates. Phytother. Res. 2014, 28, 1014–1021. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Cui, C.-F.; Yang, L.; Wang, L.; Jiang, X.-H. Emodin Inhibits Colon Cancer Cell Invasion and Migration by Suppressing Epithelial-Mesenchymal Transition via the Wnt/β-Catenin Pathway. Oncol. Res. Featur. Preclin. Clin. Cancer Ther. 2019, 27, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Yuan, Y.; Chang, P.; Li, D.; Liu, Z.; Qu, Y. Combination of aloe-emodin with radiation enhances radiation effects and improves differentiation in human cervical cancer cells. Mol. Med. Rep. 2014, 10, 731–736. [Google Scholar] [CrossRef]
- Miller, R.C.; Murley, J.S.; Rademaker, A.W.; Woloschak, G.E.; Li, J.J.; Weichselbaum, R.R.; Grdina, D.J. Very low doses of ionizing radiation and redox associated modifiers affect survivin-associated changes in radiation sensitivity. Free Radic. Biol. Med. 2016, 99, 110–119. [Google Scholar] [CrossRef]
- Chen, R.-S.; Jhan, J.-Y.; Su, Y.-J.; Lee, W.-T.; Cheng, C.-M.; Ciou, S.-C.; Lin, S.-T.; Chuang, S.-M.; Ko, J.-C.; Lin, Y.-W. Emodin enhances gefitinib-induced cytotoxicity via Rad51 downregulation and ERK1/2 inactivation. Exp. Cell Res. 2009, 315, 2658–2672. [Google Scholar] [CrossRef]
- Lai, J.-M.; Chang, J.T.; Wen, C.-L.; Hsu, S.-L. Emodin induces a reactive oxygen species-dependent and ATM-p53-Bax mediated cytotoxicity in lung cancer cells. Eur. J. Pharmacol. 2009, 623, 1–9. [Google Scholar] [CrossRef]
- Ko, J.-C.; Su, Y.-J.; Lin, S.-T.; Jhan, J.-Y.; Ciou, S.-C.; Cheng, C.-M.; Chiu, Y.-F.; Kuo, Y.-H.; Tsai, M.-S.; Lin, Y.-W. Emodin enhances cisplatin-induced cytotoxicity via down-regulation of ERCC1 and inactivation of ERK1/2. Lung Cancer 2010, 69, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Kong, W.; Wei, J.; Abidi, P.; Lin, M.; Inaba, S.; Li, C.; Wang, Y.; Wang, Z.; Si, S.; Pan, H. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat. Med. 2004, 10, 1344–1351. [Google Scholar] [CrossRef]
- Yin, J.; Xing, H.; Ye, J. Efficacy of berberine in patients with type 2 diabetes mellitus. Metabolism 2008, 57, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, Q.; Xu, B.; Wu, J.; Guo, C.; Zhu, F.; Yang, Q.; Gao, G.; Gong, Y.; Shao, C. Berberine induces p53-dependent cell cycle arrest and apoptosis of human osteosarcoma cells by inflicting DNA damage. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2009, 662, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.-P.; Yang, J.-S.; Lee, J.-H.; Hsieh, W.-T.; Chung, J.-G. Berberine induces cell cycle arrest and apoptosis in human gastric carcinoma SNU-5 cell line. World J. Gastroenterol. 2006, 12, 21. [Google Scholar] [CrossRef]
- Hwang, J.-M.; Kuo, H.-C.; Tseng, T.-H.; Liu, J.-Y.; Chu, C.-Y. Berberine induces apoptosis through a mitochondria/caspases pathway in human hepatoma cells. Arch. Toxicol. 2006, 80, 62–73. [Google Scholar] [CrossRef]
- Peng, P.-l.; Kuo, W.-H.; Tseng, H.-C.; Chou, F.-P. Synergistic tumor-killing effect of radiation and berberine combined treatment in lung cancer: The contribution of autophagic cell death. Int. J. Radiat. Oncol. Biol. Phys. 2008, 70, 529–542. [Google Scholar] [CrossRef]
- Liu, Q.; Jiang, H.; Liu, Z.; Wang, Y.; Zhao, M.; Hao, C.; Feng, S.; Guo, H.; Xu, B.; Yang, Q. Berberine radiosensitizes human esophageal cancer cells by downregulating homologous recombination repair protein RAD51. PLoS ONE 2011, 6, e23427. [Google Scholar] [CrossRef]
- Rosser, C.J.; Tanaka, M.; Pisters, L.L.; Tanaka, N.; Levy, L.B.; Hoover, D.C.; Grossman, H.B.; McDonnell, T.J.; Kuban, D.A.; Meyn, R.E. Adenoviral-mediated PTEN transgene expression sensitizes Bcl-2-expressing prostate cancer cells to radiation. Cancer Gene Ther. 2004, 11, 273–279. [Google Scholar] [CrossRef]
- Szotowski, B.; Antoniak, S.; Goldin-Lang, P.; Tran, Q.-V.; Pels, K.; Rosenthal, P.; Bogdanov, V.Y.; Borchert, H.-H.; Schultheiss, H.-P.; Rauch, U. Antioxidative treatment inhibits the release of thrombogenic tissue factor from irradiation-and cytokine-induced endothelial cells. Cardiovasc. Res. 2007, 73, 806–812. [Google Scholar] [CrossRef]
- Böhnke, A.; Westphal, F.; Schmidt, A.; El-Awady, R.; Dahm-Daphi, J. Role of p53 mutations, protein function and DNA damage for the radiosensitivity of human tumour cells. Int. J. Radiat. Biol. 2004, 80, 53–63. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, F. Reactive oxygen species (ROS), troublemakers between nuclear factor-κB (NF-κB) and c-Jun NH2-terminal kinase (JNK). Cancer Res. 2004, 64, 1902–1905. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yang, Q. Radiosensitization effects of berberine on human breast cancer cells. Int. J. Mol. Med. 2012, 30, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Kawano, M.; Takagi, R.; Kaneko, A.; Matsushita, S.J. Berberine is a dopamine D1-and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J. Neuroimmunol. 2015, 289, 43–55. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.L.; Zhang, M.; Xu, H.; Wang, C.C.; Wang, S.; Duan, R.S. Berberine ameliorates experimental autoimmune neuritis by suppressing both cellular and humoral immunity. Scand. J. Immunol. 2014, 79, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.X.; Pang, W.Q.; Zeng, Q.X.; Deng, Z.S.; Fan, T.Y.; Jiang, J.D.; Deng, H.B.; Song, D.Q. Synthesis and biological evaluation of new berberine derivatives as cancer immunotherapy agents through targeting IDO1. Eur. J. Med. Chem. 2018, 143, 1858–1868. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B. Pathophysiological significance of protein hydrophobic interactions: An emerging hypothesis. Med. Hypotheses 2018, 110, 15–22. [Google Scholar] [CrossRef]
- Kieliszek, M.; Lipinski, B.; Błażejak, S. Application of sodium selenite in the prevention and treatment of cancers. Cells 2017, 6, 39. [Google Scholar] [CrossRef]
- Liu, T.; Shi, C.; Duan, L.; Zhang, Z.; Luo, L.; Goel, S.; Cai, W.; Chen, T. A highly hemocompatible erythrocyte membrane-coated ultrasmall selenium nanosystem for simultaneous cancer radiosensitization and precise antiangiogenesis. J. Mater. Chem. B 2018, 6, 4756–4764. [Google Scholar] [CrossRef]
- Wang, M.; Law, M.E.; Castellano, R.K.; Law, B.K. The unfolded protein response as a target for anticancer therapeutics. Crit. Rev. Oncol. 2018, 127, 66–79. [Google Scholar] [CrossRef] [PubMed]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Uifălean, A.; Schneider, S.; Ionescu, C.; Lalk, M.; Iuga, C.A. Soy isoflavones and breast cancer cell lines: Molecular mechanisms and future perspectives. Molecules 2016, 21, 13. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol. Cell 2002, 9, 459–470. [Google Scholar] [CrossRef]
- Altieri, D.C. Validating survivin as a cancer therapeutic target. Nat. Rev. Cancer 2003, 3, 46–54. [Google Scholar] [CrossRef]
- Zhang, B.; Liu, J.-y.; Pan, J.-s.; Han, S.-p.; Yin, X.-x.; Wang, B.; Hu, G. Combined treatment of ionizing radiation with genistein on cervical cancer HeLa cells. J. Pharmacol. Sci. 2006, 102, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Yashar, C.M.; Spanos, W.J.; Taylor, D.D.; Gercel-Taylor, C. Potentiation of the radiation effect with genistein in cervical cancer cells. Gynecol. Oncol. 2005, 99, 199–205. [Google Scholar] [CrossRef]
- Wang, S.; Yang, K.; Hsu, Y.; Chang, C.; Yang, Y. The differential inhibitory effects of genistein on the growth of cervical cancer cells in vitro. Neoplasma 2001, 48, 227–233. [Google Scholar]
- Liu, H.-Y.; Skjetne, E.; Kobernus, M. Mobile phone tracking: In support of modelling traffic-related air pollution contribution to individual exposure and its implications for public health impact assessment. Environ. Health 2013, 12, 93. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bai, Q.; Zou, L.Y.; Zhang, Q.Y.; Zhou, Y.; Chang, H.; Yi, L.; Zhu, J.D.; Mi, M.T. Genistein inhibits DNA methylation and increases expression of tumor suppressor genes in human breast cancer cells. Genes Chromosomes Cancer 2014, 53, 422–431. [Google Scholar] [CrossRef]
- Majid, S.; Dar, A.A.; Shahryari, V.; Hirata, H.; Ahmad, A.; Saini, S.; Tanaka, Y.; Dahiya, A.V.; Dahiya, R. Genistein reverses hypermethylation and induces active histone modifications in tumor suppressor gene B-Cell translocation gene 3 in prostate cancer. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2010, 116, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Baird, L.; Llères, D.; Swift, S.; Dinkova-Kostova, A.T. Regulatory flexibility in the Nrf2-mediated stress response is conferred by conformational cycling of the Keap1-Nrf2 protein complex. Proc. Natl. Acad. Sci. USA 2013, 110, 15259–15264. [Google Scholar] [CrossRef]
- Cho, H.-Y.; Reddy, S.P.; DeBiase, A.; Yamamoto, M.; Kleeberger, S.R. Gene expression profiling of NRF2-mediated protection against oxidative injury. Free Radic. Biol. Med. 2005, 38, 325–343. [Google Scholar] [CrossRef]
- Liu, X.; Sun, C.; Liu, B.; Jin, X.; Li, P.; Zheng, X.; Zhao, T.; Li, F.; Li, Q. Genistein mediates the selective radiosensitizing effect in NSCLC A549 cells via inhibiting methylation of the keap1 gene promoter region. Oncotarget 2016, 7, 27267. [Google Scholar] [CrossRef]
- You, S.; Li, R.; Park, D.; Xie, M.; Sica, G.L.; Cao, Y.; Xiao, Z.-Q.; Deng, X. Disruption of STAT3 by niclosamide reverses radioresistance of human lung cancer. Mol. Cancer Ther. 2014, 13, 606–616. [Google Scholar] [CrossRef]
- Du, Y.; Ji, X. Bcl-2 down-regulation by small interfering RNA induces Beclin1-dependent autophagy in human SGC-7901 cells. Cell Biol. Int. 2014, 38, 1155–1162. [Google Scholar] [CrossRef] [PubMed]
- Pattingre, S.; Tassa, A.; Qu, X.; Garuti, R.; Liang, X.H.; Mizushima, N.; Packer, M.; Schneider, M.D.; Levine, B. Bcl-2 antiapoptotic proteins inhibit Beclin 1-dependent autophagy. Cell 2005, 122, 927–939. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Jin, F.; Lian, X.; Li, M.; Wang, G.; Lan, B.; He, H.; Liu, G.-D.; Wu, Y.; Sun, G. Genistein promotes ionizing radiation-induced cell death by reducing cytoplasmic Bcl-xL levels in non-small cell lung cancer. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Yenice, E.; Bilir, B.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ozercan, I.H.; Kabil, N.; Ozpolat, B.; Kucuk, O.J. Genistein prevents development of spontaneous ovarian cancer and inhibits tumor growth in hen model. Cancer Prev. Res. 2019, 12, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, Y.C.; Peng, S.F.; Lai, K.C.; Liao, C.L.; Huang, Y.P.; Lin, C.C.; Lin, M.L.; Liu, K.C.; Tsai, C.C.; Ma, Y.S. Genistein induces apoptosis in vitro and has antitumor activity against human leukemia HL-60 cancer cell xenograft growth in vivo. Environ. Toxicol. 2019, 34, 443–456. [Google Scholar] [CrossRef]
- Holsti, L.R. Development of clinical radiotherapy since 1896. Acta Oncol. 1995, 34, 995–1003. [Google Scholar] [CrossRef]
- Gai, S.; Yang, G.; Yang, P.; He, F.; Lin, J.; Jin, D.; Xing, B. Recent advances in functional nanomaterials for light–triggered cancer therapy. Nano Today 2018, 19, 146–187. [Google Scholar] [CrossRef]
- Alberti, C. Organ-confined prostate carcinoma radiation brachytherapy compared with external either photon-or hadron-beam radiation therapy. Just a short up-to-date. Eur. Rev. Med. Pharmacol. Sci. 2011, 15, 769–774. [Google Scholar]
- Islam, M.A.; Islam, S.; Akter, A.; Rahman, M.H.; Nandwani, D. Effect of organic and inorganic fertilizers on soil properties and the growth, yield and quality of tomato in Mymensingh, Bangladesh. Agriculture 2017, 7, 18. [Google Scholar] [CrossRef]
- Malfa, G.A.; Tomasello, B.; Acquaviva, R.; Genovese, C.; La Mantia, A.; Cammarata, F.P.; Ragusa, M.; Renis, M.; Di Giacomo, C. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int. J. Mol. Sci. 2019, 20, 2723. [Google Scholar] [CrossRef]
- Reed, D.; Raina, K.; Agarwal, R. Nutraceuticals in prostate cancer therapeutic strategies and their neo-adjuvant use in diverse populations. NPJ Precis. Oncol. 2018, 2, 1–20. [Google Scholar] [CrossRef]
- Das, L.; Bhaumik, E.; Raychaudhuri, U.; Chakraborty, R. Role of nutraceuticals in human health. J. Food Sci. Technol. 2012, 49, 173–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.E.; Ivanov, V.N.; Hei, T.K. Radiosensitization of melanoma cells through combined inhibition of protein regulators of cell survival. Apoptosis 2008, 13, 790. [Google Scholar] [CrossRef] [PubMed]
- Minafra, L.; Porcino, N.; Bravatà, V.; Gaglio, D.; Bonanomi, M.; Amore, E.; Cammarata, F.P.; Russo, G.; Militello, C.; Savoca, G. Radiosensitizing effect of curcumin-loaded lipid nanoparticles in breast cancer cells. Sci. Rep. 2019, 9, 1–16. [Google Scholar]
- Sandur, S.K.; Deorukhkar, A.; Pandey, M.K.; Pabón, A.M.; Shentu, S.; Guha, S.; Aggarwal, B.B.; Krishnan, S. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-κB activity. Int. J. Radiat. Oncol. Biol. Phys. 2009, 75, 534–542. [Google Scholar] [CrossRef]
- Spina, C.; Tangpricha, V.; Yao, M.; Zhou, W.; Wolfe, M.; Maehr, H.; Uskokovic, M.; Adorini, L.; Holick, M.J. Colon cancer and solar ultraviolet B radiation and prevention and treatment of colon cancer in mice with vitamin D and its Gemini analogs. J. Steroid. Biochem. Mol. Biol. 2005, 97, 111–120. [Google Scholar] [CrossRef]
- Chlebowski, R.T.; Johnson, K.C.; Kooperberg, C.; Pettinger, M.; Wactawski-Wende, J.; Rohan, T.; Rossouw, J.; Lane, D.; O’Sullivan, M.J.; Yasmeen, S.J. Calcium plus vitamin D supplementation and the risk of breast cancer. J. Natl. Cancer Inst. 2008, 100, 1581–1591. [Google Scholar] [CrossRef]
- Dai, Y.; DeSano, J.T.; Meng, Y.; Ji, Q.; Ljungman, M.; Lawrence, T.S.; Xu, L. Celastrol potentiates radiotherapy by impairment of DNA damage processing in human prostate cancer. Int. J. Radiat. Oncol. Biol. Phys. 2009, 74, 1217–1225. [Google Scholar] [CrossRef]
- Chiang, M.-F.; Chen, H.-H.; Chi, C.-W.; Sze, C.-I.; Hsu, M.-L.; Shieh, H.-R.; Lin, C.-P.; Tsai, J.-T.; Chen, Y.-J. Modulation of Sonic hedgehog signaling and WW domain containing oxidoreductase WOX1 expression enhances radiosensitivity of human glioblastoma cells. Exp. Biol. Med. 2015, 240, 392–399. [Google Scholar] [CrossRef]
- Deorukhkar, A.; Ahuja, N.; Mercado, A.L.; Diagaradjane, P.; Raju, U.; Patel, N.; Mohindra, P.; Diep, N.; Guha, S.; Krishnan, S. Zerumbone increases oxidative stress in a thiol-dependent ROS-independent manner to increase DNA damage and sensitize colorectal cancer cells to radiation. Cancer Med. 2015, 4, 278–292. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, M.; Hu, J.; Lv, X.; Yu, L.; Qian, X.; Liu, B. Enhancement of radiation effects by ursolic acid in BGC-823 human adenocarcinoma gastric cancer cell line. PLoS ONE 2015, 10, e0133169. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.; Kim, D. Berberine inhibited radioresistant effects and enhanced anti-tumor effects in the irradiated-human prostate cancer cells. Toxicol. Res. 2010, 26, 109–115. [Google Scholar] [CrossRef] [PubMed]


| Natural Products | Chemical Structure | Target of Tumors | Types of Treatment | Mechanism of Action | References |
|---|---|---|---|---|---|
| Resveratrol |  | Breast Cancer, Glioblastoma, Head and Neck squamous Cancer, Melanoma, Nasopharyngeal | γ-rays X-rays | Help to cure breast cancer | [21,24,163] |
| Curcumin |  | Breast cancer, Colonrectal Cancer | γ-rays X-rays | Help to reduce colonrectal cancer and breast cancer | [164,165] |
| Vitamin D |  | Colon and breast cancer | ----- | Help to cure colon cancer | [166,167] |
| Celastrol |  | Prostate Cancer | γ-rays X-rays | Help to cure c prostate cancer | [168] |
| Zerumbone |  | Glioblastoma, Colonrectal Cancer | γ-rays X-rays | Curecolonrectal cancer | [169,170] |
| Ursolic Acid |  | Gastric Cancer | γ-rays X-rays | Help to reduce gastric cancer | [171] |
| Withaferin A |  | Carcinoma | γ-rays X-rays | CureCarcinoa | [97] |
| Emodin |  | Carcinoma, cervical cancer | γ-rays X-rays | Help to reduce cervical cancer | [112] |
| Berberine |  | Prostate cancer, Human breast cancer | γ-rays X-rays | Help to reduce prostate cancer, human breast cancer | [128,172] |
| Selenium | Se | Cancer cells | γ-rays X-rays | Eliminate cancer cells | [132,133] |
| Genistein |  | Cervical Cancer | γ-rays X-rays | Help to reducecervical cancer | [142,153] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akter, R.; Najda, A.; Rahman, M.H.; Shah, M.; Wesołowska, S.; Hassan, S.S.u.; Mubin, S.; Bibi, P.; Saeeda, S. RETRACTED: Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives. Molecules 2021, 26, 5997. https://doi.org/10.3390/molecules26195997
Akter R, Najda A, Rahman MH, Shah M, Wesołowska S, Hassan SSu, Mubin S, Bibi P, Saeeda S. RETRACTED: Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives. Molecules. 2021; 26(19):5997. https://doi.org/10.3390/molecules26195997
Chicago/Turabian StyleAkter, Rokeya, Agnieszka Najda, Md. Habibur Rahman, Muddaser Shah, Sylwia Wesołowska, Syed Shams ul Hassan, Sidra Mubin, Parveen Bibi, and Saeeda Saeeda. 2021. "RETRACTED: Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives" Molecules 26, no. 19: 5997. https://doi.org/10.3390/molecules26195997
APA StyleAkter, R., Najda, A., Rahman, M. H., Shah, M., Wesołowska, S., Hassan, S. S. u., Mubin, S., Bibi, P., & Saeeda, S. (2021). RETRACTED: Potential Role of Natural Products to Combat Radiotherapy and Their Future Perspectives. Molecules, 26(19), 5997. https://doi.org/10.3390/molecules26195997