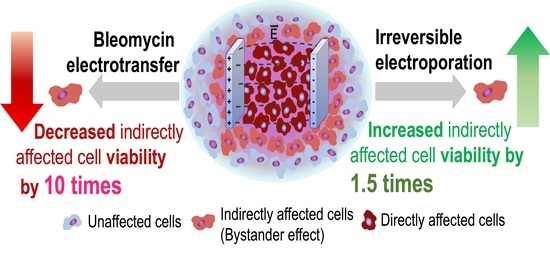
The Evidence of the Bystander Effect after Bleomycin Electrotransfer and Irreversible Electroporation
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Cell Electroporation
4.3. Cell Viability Evaluation
4.3.1. Viability Evaluation of Directly Affected Cells after the Bleomycin Electrotransfer or Irreversible Electroporation
4.3.2. Viability Evaluation of Indirectly Affected Cells after Bleomycin Electrotransfer or Irreversible Electroporation
4.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Peng, Y.; Tao, H.; Gao, Y.; Yang, Y.; Chen, Z. Review and Prospect of Tissue-agnostic Targeted Strategies in Anticancer Therapies. Curr. Top. Med. Chem. 2020, 21, 404–425. [Google Scholar] [CrossRef]
- Pirc, E.; Federici, C.; Bošnjak, M.; Perić, B.; Reberšek, M.; Pecchia, L.; Glumac, N.; Čemažar, M.; Snoj, M.; Serša, G.; et al. Early Cost-effectiveness Analysis of Electrochemotherapy as a Prospect Treatment Modality for Skin Melanoma. Clin. Ther. 2020, 42, 1535–1548.e2. [Google Scholar] [CrossRef] [PubMed]
- Fini, M.; Salamanna, F.; Parrilli, A.; Martini, L.; Cadossi, M.; Maglio, M.; Borsari, V. Electrochemotherapy is effective in the treatment of rat bone metastases. Clin. Exp. Metastasis 2013, 30, 1033–1045. [Google Scholar] [CrossRef] [PubMed]
- Ricotti, F.; Giuliodori, K.; Cataldi, I.; Campanati, A.; Ganzetti, G.; Ricotti, G.; Offidani, A. Electrochemotherapy: An effective local treatment of cutaneous and subcutaneous melanoma metastases. Dermatol. Ther. 2014, 27, 148–152. [Google Scholar] [CrossRef] [PubMed]
- Šatkauskas, S.; Ruzgys, P.; Venslauskas, M.S. Towards the mechanisms for efficient gene transfer into cells and tissues by means of cell electroporation. Expert Opin. Biol. Ther. 2012, 12, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Scheffer, H.J.; Graybill, P.M.; Ruarus, A.H.; Nieuwenhuizen, S.; Puijk, R.S.; Van Den Tol, P.M.; Davalos, R.V.; Rubinsky, B.; De Gruijl, T.D.; et al. High-voltage electrical pulses in oncology: Irreversible electroporation, electrochemotherapy, gene electrotransfer, electrofusion, and electroimmunotherapy. Radiology 2020, 295, 254–272. [Google Scholar] [CrossRef]
- Escoffre, J.-M.; Rols, M.-P. Electrochemotherapy: Progress and prospects. Curr. Pharm. Des. 2012, 18, 3406–3415. [Google Scholar] [CrossRef]
- Probst, U.; Fuhrmann, I.; Beyer, L.; Wiggermann, P. Electrochemotherapy as a new modality in interventional oncology: A review. Technol. Cancer Res. Treat. 2018, 17, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gehl, J.; Sersa, G.; Matthiessen, L.W.; Muir, T.; Occhini, A.; Quaglino, P.; Curatolo, P.; Campana, L.G.; Kunte, C.; Clover, A.J.P.; et al. Updated standard operating procedures for electrochemotherapy of cutaneous tumours and skin metastases. Acta Oncol. 2018, 57, 874–882. [Google Scholar] [CrossRef]
- Marty, M.; Sersa, G.; Garbay, J.R.; Gehl, J.; Collins, C.G.; Snoj, M.; Billard, V.; Geertsen, P.F.; Larkin, J.O.; Miklavcic, D.; et al. Electrochemotherapy–An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: Results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Eur. J. Cancer Suppl. 2006, 4, 3–13. [Google Scholar] [CrossRef]
- Hecht, S.M. Bleomycin: New perspectives on the mechanism of action. J. Nat. Prod. 2000, 63, 158–168. [Google Scholar] [CrossRef]
- Bonnafous, P.; Vernhes, M.C.; Teissié, J.; Gabriel, B. The generation of reactive-oxygen species associated with long-lasting pulse-induced electropermeabilisation of mammalian cells is based on a non-destructive alteration of the plasma membrane. Biochim. Biophys. Acta 1999, 1461, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Berton, M.; Mir, L.M. Investigation of the chemical mechanisms involved in the electropulsation of membranes at the molecular level. Bioelectrochemistry 2018, 119, 76–83. [Google Scholar] [CrossRef]
- Azan, A.; Gailliegue, F.; Mir, L.M.; Berton, M. Cell Membrane Electropulsation: Chemical Analysis of Cell Membrane Modifications and Associated Transport Mechanisms. Adv. Anat. Embryol. Cell Biol. 2017, 227, 59–71. [Google Scholar]
- Campana, L.G.; Sundararajan, R.; Chiarion-Sileni, V.; Rossi, C.R. Clinical electrochemotherapy for advanced superficial melanoma. Electroporation-Based Ther. Cancer 2014. [Google Scholar] [CrossRef]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar] [CrossRef]
- Roh, J.S.; Sohn, D.H. Damage-Associated Molecular Patterns in Inflammatory Diseases. Immune Netw. 2018, 18, 4. [Google Scholar] [CrossRef] [PubMed]
- Solari, J.I.G.; Filippi-Chiela, E.; Pilar, E.S.; Nunes, V.; Gonzalez, E.A.; Figueiró, F.; Andrade, C.F.; Klamt, F. Damage-associated molecular patterns (DAMPs) related to immunogenic cell death are differentially triggered by clinically relevant chemotherapeutics in lung adenocarcinoma cells. BMC Cancer 2020, 20, 474. [Google Scholar] [CrossRef] [PubMed]
- Krysko, O.; Aaes, T.L.; Bachert, C.; Vandenabeele, P.; Krysko, D.V. Many faces of DAMPs in cancer therapy. Cell Death Dis. 2013, 4, e631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvet, C.Y.; Famin, D.; André, F.M.; Lluis, M.M. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014, 3, e28131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Wen, X.; Tian, L.; Li, T.; Xu, C.; Wen, X.; Melancon, M.P.; Gupta, S.; Shen, B.; Peng, W.; et al. Irreversible electroporation reverses resistance to immune checkpoint blockade in pancreatic cancer. Nat. Commun. 2019, 10, 899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.M.; Choi, H.S.; Kim, E.S.; Keum, B.; Seo, Y.S.; Jeen, Y.T.; Lee, H.S.; Chun, H.J.; Um, S.H.; Kim, C.D.; et al. Characterization of irreversible electroporation on the stomach: A feasibility study in rats. Sci. Rep. 2019, 9, 9094. [Google Scholar] [CrossRef]
- Szlasa, W.; Kiełbik, A.; Szewczyk, A.; Rembiałkowska, N.; Novickij, V.; Tarek, M.; Saczko, J.; Kulbacka, J. Oxidative Effects during Irreversible Electroporation of Melanoma Cells—In Vitro Study. Molecules. 2020, 26, 154. [Google Scholar] [CrossRef]
- Jakstys, B.; Jakutaviciute, M.; Uzdavinyte, D.; Satkauskiene, I.; Satkauskas, S. Correlation between the loss of intracellular molecules and cell viability after cell electroporation. Bioelectrochemistry 2020, 135. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.; Her, S.; Jaffray, D.A. Radiotherapy for Cancer: Present and Future. Adv. Drug Deliv. Rev. 2017, 109, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, U.S.; Tan, B.W.Q.; Vellayappan, B.A.; Jeyasekharan, A.D. ROS and the DNA damage response in cancer. Redox Biol. 2019, 25, 101084. [Google Scholar] [CrossRef]
- Zou, Z.; Chang, H.; Li, H.; Wang, S. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Georgakilas, A.G. Bystander and non-targeted effects: A unifying model from ionizing radiation to cancer. Cancer Lett. 2015, 356, 3–4. [Google Scholar] [CrossRef]
- Jalal, N.; Haq, S.; Anwar, N.; Nazeer, S.; Saeed, U. Radiation induced bystander effect and DNA damage. J. Cancer Res. Ther. 2014, 10, 819–833. [Google Scholar] [CrossRef]
- Dong, S.; Lyu, X.; Yuan, S.; Wang, S.; Li, W.; Chen, Z.; Yu, H.; Li, F.; Jiang, Q. Oxidative stress: A critical hint in ionizing radiation induced pyroptosis. Radiat. Med. Prot. 2020, 1, 179–185. [Google Scholar] [CrossRef]
- Rödel, F.; Frey, B.; Multhoff, G.; Gaipl, U. Contribution of the immune system to bystander and non-targeted effects of ionizing radiation. Cancer Lett. 2015, 356, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Furlong, H.; Mothersill, C.; Lyng, F.M.; Howe, O. Apoptosis is signalled early by low doses of ionising radiation in a radiation-induced bystander effect. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2013, 741–742, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Jakštys, B.; Ruzgys, P.; Tamošiūnas, M.; Šatkauskas, S. Different Cell Viability Assays Reveal Inconsistent Results After Bleomycin Electrotransfer In Vitro. J. Membr. Biol. 2015, 248, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Gehl, J.; Skovsgaard, T.; Mir, L.M. Enhancement of cytotoxicity by electropermeabilization: An improved method for screening drugs. Anticancer. Drugs 1998, 9, 319–325. [Google Scholar] [CrossRef]
- Ruzgys, P.; Jakutaviciute, M.; Chopra, S.; Satkauskas, S. Enhancement of drug electrotransfer by extracellular plasmid DNA. Arch. Biochem. Biophys. 2019, 666, 156–160. [Google Scholar] [CrossRef]
- Marín, A.; Martín, M.; Liñán, O.; Alvarenga, F.; López, M.; Fernández, L.; Büchser, D.; Cerezo, L. Bystander effects and radiotherapy. Reports Pract. Oncol. Radiother. 2015, 20, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Morgan, W.F. Non-targeted and Delayed Effects of Exposure to Ionizing Radiation: II. Radiation-Induced Genomic Instability and Bystander Effects In Vivo, Clastogenic Factors and Transgenerational Effects. Radiat. Res. 2003, 159, 581–596. [Google Scholar] [CrossRef]
- Rotunno, R.; Campana, L.G.; Quaglino, P.; Terlizzi, F.; Kunte, C.; Odili, J.; Gehl, J.; Ribero, S.; Liew, S.H.; Marconato, R.; et al. Electrochemotherapy of unresectable cutaneous tumours with reduced dosages of intravenous bleomycin: Analysis of 57 patients from the International Network for Sharing Practices of Electrochemotherapy registry. J. Eur. Dermatol. Venereol. 2017, 32, 1147–1154. [Google Scholar] [CrossRef]
- Fantini, F.; Gualdi, G.; Cimitan, A.; Giannetti, A. Metastatic basal cell carcinoma with squamous differentiation: Report of a case with response of cutaneous metastases to electrochemotherapy. Arch. Dermatol. 2008, 144, 1186–1188. [Google Scholar] [CrossRef] [Green Version]
- Calafat, A.M.; Won, H.; Marzilli, L.G. A new arrangement for the anticancer antibiotics tallysomycin and bleomycin when bound to zinc: An assessment of metal and ligand chirality by NMR and molecular dynamics. J. Am. Chem. Soc. 1997, 119, 3656–3664. [Google Scholar] [CrossRef]
- Chen, J.; Ghorai, M.K.; Kenney, G.; Stubbe, J. Mechanistic studies on bleomycin-mediated DNA damage: Multiple binding modes can result in double-stranded DNA cleavage. Nucleic Acids Res. 2008, 36, 3781–3790. [Google Scholar] [CrossRef] [Green Version]
- Hamamichi, N.; Hecht, S.M. Determination of the Absolute Configuration of the Thiazolinylthiazole Moiety of Phleomycin. J. Am. Chem. Soc. 1993, 115, 12605–12606. [Google Scholar] [CrossRef]
- Chopra, S.; Ruzgys, P.; Jakutaviciute, M.; Rimgailaite, A.; Navickaitė, D.; Satkauskas, S. A novel method for controlled gene expression via combined Bleomycin and Plasmid DNA Electrotransfer. Int. J. Mol. Sci. 2019, 20, 4047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Azzam, E.I.; De Toledo, S.M.; Little, J.B. Oxidative metabolism, gap junctions and the ionizing radiation-induced bystander effect. Oncogene 2003, 22, 7050–7057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mosmann, T.R. Properties and functions of interleukin-10. Adv. Immunol. 1994, 56, 1–26. [Google Scholar] [PubMed]
- Dinarello, C.A. A clinical perspective of IL-1β as the gatekeeper of inflammation. Eur. J. Immunol. 2011, 41, 1203–1217. [Google Scholar] [CrossRef]
- Baggiolini, M.; Loetscher, P. Chemokines in inflammation and immunity. Immunol. Today 2000, 21, 418–420. [Google Scholar] [CrossRef]
- Georgieva, M.; Stoilov, L. Assessment of DNA strand breaks induced by bleomycin in barley by the comet assay. Environ. Mol. Mutagen. 2008, 49, 381–387. [Google Scholar] [CrossRef]
- Blagosklonny, M.V. Mitotic arrest and cell fate: Why and how mitotic inhibition of transcription drives mutually exclusive events. Cell Cycle 2007, 6, 70–74. [Google Scholar] [CrossRef]
- Pomp, J.; Wike, J.L.; Ouwerkerk, I.J.M.; Hoogstraten, C.; Davelaar, J.; Schrier, P.I.; Leer, J.W.H.; Thames, H.D.; Brock, W.A. Cell density dependent plating efficiency affects outcome and interpretation of colony forming assays. Radiother. Oncol. 1996, 40, 121–125. [Google Scholar] [CrossRef]
- Chow, M.T.; Luster, A.D. Chemokines in cancer. Cancer Immunol. Res. 2014, 2, 1125–1131. [Google Scholar] [CrossRef] [Green Version]
- Bhat, A.A.; Nisar, S.; Maacha, S.; Carneiro-Lobo, T.C.; Akhtar, S.; Siveen, K.S.; Wani, N.A.; Rizwan, A.; Bagga, P.; Singh, M.; et al. Cytokine-chemokine network driven metastasis in esophageal cancer; promising avenue for targeted therapy. Mol. Cancer 2021, 20, 2. [Google Scholar] [CrossRef]
- Prevc, A.; Bedina Zavec, A.; Cemazar, M.; Kloboves-Prevodnik, V.; Stimac, M.; Todorovic, V.; Strojan, P.; Sersa, G. Bystander Effect Induced by Electroporation is Possibly Mediated by Microvesicles and Dependent on Pulse Amplitude, Repetition Frequency and Cell Type. J. Membr. Biol. 2016, 249, 703–711. [Google Scholar] [CrossRef]
- Corovic, S.; Lackovic, I.; Sustaric, P.; Sustar, T.; Rodic, T.; Miklavcic, D. Modeling of electric field distribution in tissues during electroporation. Biomed. Eng. Online 2013, 12, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marino, M.; Luján, E.; Mocskos, E.; Marshall, G. OpenEP: An open-source simulator for electroporation-based tumor treatments. Sci. Rep. 2021, 11, 1423. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Bagga, M.; Kaur, A.; Westermarck, J.; Abankwa, D. ColonyArea: An ImageJ Plugin to Automatically Quantify Colony Formation in Clonogenic Assays. PLoS ONE 2014, 9, e92444. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ruzgys, P.; Barauskaitė, N.; Novickij, V.; Novickij, J.; Šatkauskas, S. The Evidence of the Bystander Effect after Bleomycin Electrotransfer and Irreversible Electroporation. Molecules 2021, 26, 6001. https://doi.org/10.3390/molecules26196001
Ruzgys P, Barauskaitė N, Novickij V, Novickij J, Šatkauskas S. The Evidence of the Bystander Effect after Bleomycin Electrotransfer and Irreversible Electroporation. Molecules. 2021; 26(19):6001. https://doi.org/10.3390/molecules26196001
Chicago/Turabian StyleRuzgys, Paulius, Neringa Barauskaitė, Vitalij Novickij, Jurij Novickij, and Saulius Šatkauskas. 2021. "The Evidence of the Bystander Effect after Bleomycin Electrotransfer and Irreversible Electroporation" Molecules 26, no. 19: 6001. https://doi.org/10.3390/molecules26196001
APA StyleRuzgys, P., Barauskaitė, N., Novickij, V., Novickij, J., & Šatkauskas, S. (2021). The Evidence of the Bystander Effect after Bleomycin Electrotransfer and Irreversible Electroporation. Molecules, 26(19), 6001. https://doi.org/10.3390/molecules26196001