High Substitution Synthesis of Carboxymethyl Chitosan for Properties Improvement of Carboxymethyl Chitosan Films Depending on Particle Sizes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemical Structure
2.2. Morphology of Chitosan and CMCh Powders
2.3. Yield of CMCh Powders
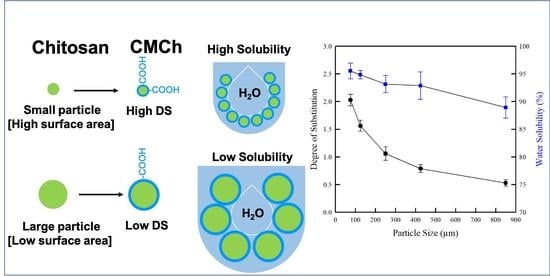
2.4. DS and Water Solubility of CMCh Powders
2.5. Morphology of CMCh Films
2.6. Mechanical Properties of CMCh Films
2.7. Contact Angle of CMCh Films
2.8. Water Solubility and Water Vapor Transmission Rate of CMCh Films
3. Materials and Methods
3.1. Materials
3.2. Synthesis of CMCh Powders
3.3. Preparation of CMCh Films
3.4. Characterizations
3.4.1. Infrared Spectroscopy
3.4.2. Morphology of Powders
3.4.3. DS
3.4.4. Water Solubility of CMCh Powders
3.4.5. Morphology of CMCh Films
3.4.6. Mechanical Properties of CMCh Films
3.4.7. Water Solubility of CMCh Films
3.4.8. Contact Angle of CMCh Films
3.4.9. WVTR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ali, A.; Ahmed, S. Recent advances in edible polymer based hydrogels as a sustainable alternative to conventional polymers. J. Agric. Food Chem. 2018, 66, 6940–6967. [Google Scholar] [CrossRef]
- Bisht, B.; Lohani, U.; Kumar, V.; Gururani, P.; Sinhmar, R. Edible hydrocolloids as sustainable substitute for non-biodegradable materials. Crit. Rev. Food Sci. Nutr. 2020, 1–33. [Google Scholar] [CrossRef]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Wu, Y.; Rashidpour, A.; Almajano, M.P.; Metón, I. Chitosan-based drug delivery system: Applications in fish biotechnology. Polymers 2020, 12, 1177. [Google Scholar] [CrossRef]
- Sakthiguru, N.; Sithique, M.A. Fabrication of bioinspired chitosan/gelatin/allantoin biocomposite film for wound dressing application. Int. J. Biol. Macromol. 2020, 152, 873–883. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and cellulose-based hydrogels for wound management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Anchisi, C.; Meloni, M.; Maccioni, A.M. Chitosan beads loaded with essential oils in cosmetic formulations. J. Cosmet. Sci. 2006, 57, 205–214. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Qian, J.; Ding, F. Emerging chitosan-based films for food packaging applications. J. Agric. Food Chem. 2018, 66, 395–413. [Google Scholar] [CrossRef] [PubMed]
- Santos, V.P.; Marques, N.S.; Maia, P.C.; Lima, M.A.B.D.; Franco, L.D.O.; Campos-Takaki, G.M.D. Seafood waste as attractive source of chitin and chitosan production and their applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekharan, A.; Hwang, Y.J.; Seong, K.-Y.; Park, S.; Kim, S.; Yang, S.Y. Acid-treated water-soluble chitosan suitable for microneedle-assisted intracutaneous drug delivery. Pharmaceutics 2019, 11, 209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bukzem, A.L.; Signini, R.; Dos Santos, D.M.; Lião, L.M.; Ascheri, D.P.R. Optimization of carboxymethyl chitosan synthesis using response surface methodology and desirability function. Int. J. Biol. Macromol. 2016, 85, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, P. Improvement of mechanical properties and thermal stability of biodegradable rice starch–based films blended with carboxymethyl chitosan. Ind. Crop. Prod. 2018, 122, 37–48. [Google Scholar] [CrossRef]
- Suriyatem, R.; Auras, R.A.; Rachtanapun, C.; Rachtanapun, P. Biodegradable rice starch/carboxymethyl chitosan films with added propolis extract for potential use as active food packaging. Polymers 2018, 10, 954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Zhuang, X.P.; Liu, X.F.; Guan, Y.L.; De Yao, K. Study on antibacterial O-carboxymethylated chitosan/cellulose blend film from LiCl/N, N-dimethylacetamide solution. Polymer 2002, 43, 1541–1547. [Google Scholar] [CrossRef]
- Tantala, J.; Thongngam, M.; Rachtanapun, P.; Rachtanapun, C. Antimicrobial activity of chitosan and carboxymethyl chitosan from difference types and sources of chitosan. Ital. J. Food Sci. 2012, 24, 97–101. [Google Scholar]
- Noiwan, D.; Sutenan, K.; Yodweingchai, C.; Rachtanapun, P. Postharvest life extension of fresh-cut mango (Mangifera indica cv. Fa-Lun) using chitosan and carboxymethyl chitosan coating. J. Agric. Sci. 2018, 10, 438–446. [Google Scholar] [CrossRef]
- Wannaruemon, S.; Jimtaisong, A.; Rachtananpun, P. Sodium carboxymethyl chitosan as a fixative for eau de cologne. Trop. J. Pharm. Res. 2013, 12, 45–49. [Google Scholar] [CrossRef] [Green Version]
- Rachtanapun, P.; Suriyatem, R. Moisture sorption isotherms of soy protein isolate/carboxymethyl chitosan blend films. J. Agric. Sci. Technol. A 2012, 2, 50. [Google Scholar]
- Suriyatem, R.; Rachtanapun, C.; Raviyan, P.; Intipunya, P.; Rachtanapun, P. Investigation and modeling of moisture sorption behaviour of rice starch/carboxymethyl chitosan blend films. In Proceedings of the IOP Conference Series: Materials Science and Engineering, Beijing, China, 16–18 May 2015; p. 012080. [Google Scholar]
- Chaiwong, N.; Leelapornpisid, P.; Jantanasakulwong, K.; Rachtanapun, P.; Seesuriyachan, P.; Sakdatorn, V.; Leksawasdi, N.; Phimolsiripol, Y. Antioxidant and moisturizing properties of carboxymethyl chitosan with different molecular weights. Polymers 2020, 12, 1445. [Google Scholar] [CrossRef]
- Ding, F.; Hu, B.; Lan, S.; Wang, H. Flexographic and screen printing of carboxymethyl chitosan based edible inks for food packaging applications. Food Packag. Shelf Life 2020, 26, 100559. [Google Scholar] [CrossRef]
- Abou-Zeid, N.; Waly, A.; Kandile, N.; Rushdy, A.; El-Sheikh, M.A.; Ibrahim, H. Carboxymethylchitosan/viscose blended films: Preparation, characterization and antibacterial properties. J. Mater. Sci. Eng. Adv. Technol. 2013, 7, 93–123. [Google Scholar]
- Yeasmin, M.S.; Mondal, M.I.H. Synthesis of highly substituted carboxymethyl cellulose depending on cellulose particle size. Int. J. Biol. Macromol. 2015, 80, 725–731. [Google Scholar] [CrossRef] [PubMed]
- Stodolak-Zych, E.; Jeleń, P.; Dzierzkowska, E.; Krok-Borkowicz, M.; Zych, Ł.; Boguń, M.; Rapacz-Kmita, A.; Kolesińska, B. Modification of chitosan fibers with short peptides as a model of synthetic extracellular matrix. J. Mol. Struct. 2020, 1211, 128061. [Google Scholar] [CrossRef]
- Fernandes Queiroz, M.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs. 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Yang, Q.; Zhang, L.; Zhuo, R.; Jiang, X. Synthesis of carboxymethyl chitin in aqueous solution and its thermo-and pH-sensitive behaviors. Carbohydr. Polym. 2016, 137, 600–607. [Google Scholar] [CrossRef]
- Tan, H.; Chu, C.R.; Payne, K.A.; Marra, K.G. Injectable in situ forming biodegradable chitosan–hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Leksawasdi, N.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S. Effect of Monochloroacetic Acid on Properties of Carboxymethyl Bacterial Cellulose Powder and Film from Nata de Coco. Polymers 2021, 13, 488. [Google Scholar]
- Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S.; Jantrawut, P.; Sommano, S.R. Synthesis, characterization, and application of carboxymethyl cellulose from asparagus stalk end. Polymers 2021, 13, 81. [Google Scholar]
- Mohamed, R.R.; Seoudi, R.S.; Sabaa, M.W. Synthesis and characterization of cross-linked polyethylene glycol/carboxymethyl chitosan hydrogels. Adv. Polym. Technol. 2015, 34, 21479. [Google Scholar] [CrossRef]
- Sabaa, M.W.; Mohamed, N.A.; Mohamed, R.R.; Khalil, N.M.; Abd El Latif, S.M. Synthesis, characterization and antimicrobial activity of poly (N-vinyl imidazole) grafted carboxymethyl chitosan. Carbohydr. Polym. 2010, 79, 998–1005. [Google Scholar] [CrossRef]
- Rahman, M.; Mondal, H.; Ibrahim, M.; Yeasmin, M.; Sayeed, M.A.; Hossain, M.A.; Ahmed, M.B. Conversion of lignocellulosic corn agro-waste into cellulose derivative and its potential application as pharmaceutical excipient. Processes 2020, 8, 711. [Google Scholar] [CrossRef]
- Patel, N.K.; Sinha, V.K. Synthesis, characterization and optimization of water-soluble chitosan derivatives. Int. J. Polym. Mater. 2009, 58, 548–560. [Google Scholar] [CrossRef]
- Silcock, D. Collagen-based dressings as therapeutic agents for wound healing. In Drug-Device Combination Products; Elsevier: Amsterdam, The Netherlands, 2010; pp. 280–310. [Google Scholar]
- Rachtanapun, P.; Simasatitkul, P.; Chaiwan, W.; Watthanaworasakun, Y. Effect of sodium hydroxide concentration on properties of carboxymethyl rice starch. Int. Food. Res. J. 2012, 19, 923. [Google Scholar]
- Rachtanapun, P.; Jantrawut, P.; Klunklin, W.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Insomphun, C.; Phongthai, S. Carboxymethyl bacterial cellulose from nata de coco: Effects of NaOH. Polymers 2021, 13, 348. [Google Scholar]
- Joshi, G.; Naithani, S.; Varshney, V.; Bisht, S.S.; Rana, V.; Gupta, P. Synthesis and characterization of carboxymethyl cellulose from office waste paper: A greener approach towards waste management. Waste Manag. 2015, 38, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Pushpamalar, V.; Langford, S.J.; Ahmad, M.; Lim, Y.Y. Optimization of reaction conditions for preparing carboxymethyl cellulose from sago waste. Carbohydr. Polym. 2006, 64, 312–318. [Google Scholar] [CrossRef]
- Barai, B.K.; Singhal, R.S.; Kulkarni, P. Optimization of a process for preparing carboxymethyl cellulose from water hyacinth (Eichornia crassipes). Carbohydr. Polym. 1997, 32, 229–231. [Google Scholar] [CrossRef]
- Ismail, N.; Bono, A.; Valintinus, A.; Nilus, S.; Chng, L. Optimization of reaction conditions for preparing carboxymethyl cellulose. J. Appl. Sci. 2010, 10, 2530–2536. [Google Scholar] [CrossRef] [Green Version]
- Ge, H.-C.; Luo, D.-K. Preparation of carboxymethyl chitosan in aqueous solution under microwave irradiation. Carbohydr. Res. 2005, 340, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Du, Y.; Zeng, X. Relationships between the molecular structure and moisture-absorption and moisture-retention abilities of carboxymethyl chitosan: II. Effect of degree of deacetylation and carboxymethylation. Carbohydr. Res. 2003, 338, 333–340. [Google Scholar] [CrossRef]
- Thanakkasaranee, S.; Kim, D.; Seo, J. Preparation and characterization of poly (ether-block-amide)/polyethylene glycol composite films with temperature-dependent permeation. Polymers 2018, 10, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carraher, C.E., Jr.; Seymour, R. Structure—Property Relationships in Polymers; Springer Science & Business Media: New York, NY, USA, 2012. [Google Scholar]
- Tantala, J.; Rachtanapun, C.; Rachtanapun, P. Effect of molecular sizes, sources of chitosan and plasticizer types on properties of carboxymethyl chitosan films. Adv. Mater. Res. 2012, 506, 611–614. [Google Scholar] [CrossRef]
- Wu, J.; Zhong, F.; Li, Y.; Shoemaker, C.; Xia, W. Preparation and characterization of pullulan–chitosan and pullulan–carboxymethyl chitosan blended films. Food Hydrocoll. 2013, 30, 82–91. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Rattanapanone, N. Synthesis and characterization of carboxymethyl cellulose powder and films from Mimosa pigra. J. Appl. Polym. Sci. 2011, 122, 3218–3226. [Google Scholar] [CrossRef]
- Kim, S.R.B.; Choi, Y.-G.; Kim, J.-Y.; Lim, S.-T. Improvement of water solubility and humidity stability of tapioca starch film by incorporating various gums. J. Appl. Polym. Sci. 2015, 64, 475–482. [Google Scholar] [CrossRef]
- Wang, K.; Wang, W.; Ye, R.; Liu, A.; Xiao, J.; Liu, Y.; Zhao, Y. Mechanical properties and solubility in water of corn starch-collagen composite films: Effect of starch type and concentrations. Food Chem. 2017, 216, 209–216. [Google Scholar] [CrossRef]
- Tantala, J.; Rachtanapun, C.; Tongdeesoontorn, W.; Jantanasakulwong, K.; Rachtanapun, P. Moisture sorption isotherms and prediction models of carboxymethyl chitosan films from different sources with various plasticizers. Adv. Mater. Sci. Eng. 2019, 2019, 4082439. [Google Scholar] [CrossRef] [Green Version]
- Pirsa, S.; Aghbolagh Sharifi, K. A review of the applications of bioproteins in the preparation of biodegradable films and polymers. J. Phys. Chem. Lett. 2020, 1, 47–58. [Google Scholar]
- Rachtanapun, P.; Luangkamin, S.; Tanprasert, K.; Suriyatem, R. Carboxymethyl cellulose film from durian rind. LWT Food Sci. Technol. 2012, 48, 52–58. [Google Scholar] [CrossRef]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanakkasaranee, S.; Jantanasakulwong, K.; Phimolsiripol, Y.; Leksawasdi, N.; Seesuriyachan, P.; Chaiyaso, T.; Jantrawut, P.; Ruksiriwanich, W.; Rose Sommano, S.; Punyodom, W.; et al. High Substitution Synthesis of Carboxymethyl Chitosan for Properties Improvement of Carboxymethyl Chitosan Films Depending on Particle Sizes. Molecules 2021, 26, 6013. https://doi.org/10.3390/molecules26196013
Thanakkasaranee S, Jantanasakulwong K, Phimolsiripol Y, Leksawasdi N, Seesuriyachan P, Chaiyaso T, Jantrawut P, Ruksiriwanich W, Rose Sommano S, Punyodom W, et al. High Substitution Synthesis of Carboxymethyl Chitosan for Properties Improvement of Carboxymethyl Chitosan Films Depending on Particle Sizes. Molecules. 2021; 26(19):6013. https://doi.org/10.3390/molecules26196013
Chicago/Turabian StyleThanakkasaranee, Sarinthip, Kittisak Jantanasakulwong, Yuthana Phimolsiripol, Noppol Leksawasdi, Phisit Seesuriyachan, Thanongsak Chaiyaso, Pensak Jantrawut, Warintorn Ruksiriwanich, Sarana Rose Sommano, Winita Punyodom, and et al. 2021. "High Substitution Synthesis of Carboxymethyl Chitosan for Properties Improvement of Carboxymethyl Chitosan Films Depending on Particle Sizes" Molecules 26, no. 19: 6013. https://doi.org/10.3390/molecules26196013
APA StyleThanakkasaranee, S., Jantanasakulwong, K., Phimolsiripol, Y., Leksawasdi, N., Seesuriyachan, P., Chaiyaso, T., Jantrawut, P., Ruksiriwanich, W., Rose Sommano, S., Punyodom, W., Reungsang, A., Ngo, T. M. P., Thipchai, P., Tongdeesoontorn, W., & Rachtanapun, P. (2021). High Substitution Synthesis of Carboxymethyl Chitosan for Properties Improvement of Carboxymethyl Chitosan Films Depending on Particle Sizes. Molecules, 26(19), 6013. https://doi.org/10.3390/molecules26196013