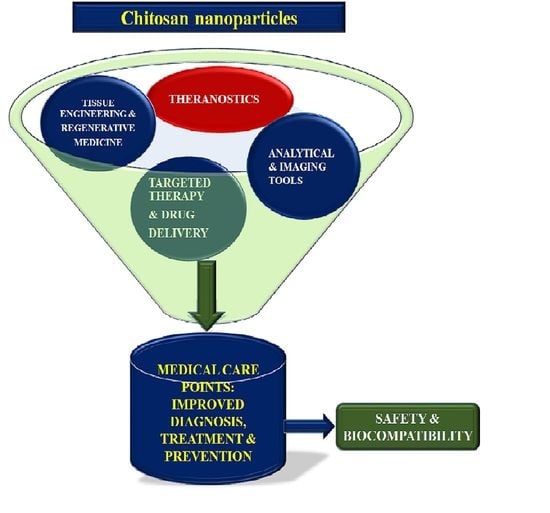
Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics
Abstract
:1. Introduction
Chitosan
2. Properties of Chitosan
2.1. Mucoadhesion
2.2. Controlled Drug Release
2.3. Permeation Enhancement
2.4. Antibacterial and Antifungal Activity
2.5. Biocompatibility and Biodegradability
3. Preparation of Chitosan NPs as Nanocarriers
3.1. Ionotropic Gelation Method
3.2. Reverse Micellar Method/Microemulsion Method
3.3. Co-Precipitation Method
3.4. Emulsion-Droplet Coalescence Method
3.5. Polyelectrolyte Complexation Method
3.6. Solvent Evaporation Method
3.7. Spray Drying Method
4. Drug Release from Chitosan NPs in Biological Fluids
4.1. Polymer Erosion/Degradation
4.2. Diffusion
4.3. Polymer Swelling
5. Properties of Chitosan NPs
6. Pharmacokinetics of Chitosan NPs
7. Safety and Toxicity of Chitosan NPs
8. Application of Chitosan NPs
8.1. CNS Diseases
8.2. Infectious Diseases
8.3. Diabetes
8.4. Antiviral Vaccines
8.5. Miscellaneous Diseases
9. Recent Developments in the Utility of Chitosan NPS
9.1. Chitosan NPs in Cancer Immunotherapy
9.2. Chitosan NPs in Theranostics
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Garg, U.; Chauhan, S.; Nagaich, U.; Jain, N. Current Advances in Chitosan Nanoparticles Based Drug Delivery and Targeting. Adv. Pharm. Bull. 2019, 9, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Bellich, B.; D’Agostino, I.; Semeraro, S.; Gamini, A.; Cesàro, A. "The Good, the Bad and the Ugly” of Chitosans. Mar. Drugs 2016, 14, 99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roy, S.G.; Shirsat, N.S.; Mishra, A.C.; Waghulde, S.O.; Kale, M.K. A review on chitosan nanoparticles applications in drug delivery. J Pharm. Phytochem. 2018, 7, 1–4. [Google Scholar] [CrossRef]
- Rizeq, B.R.; Younes, N.N.; Rasool, K.; Nasrallah, G.K. Synthesis, Bioapplications, and Toxicity Evaluation of Chitosan-Based Nanoparticles. Int. J. Mol. Sci. 2019, 20, 5776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Vimal, A.; Kumar, A. Why Chitosan? From properties to perspective of mucosal drug delivery. Int. J. Biol. Macromol. 2016, 91, 615–622. [Google Scholar] [CrossRef] [PubMed]
- Karava, A.; Lazaridou, M.; Nanaki, S.; Michailidou, G.; Christodoulou, E.; Kostoglou, M.; Iatrou, H.; Bikiaris, D.N. Chitosan Derivatives with Mucoadhesive and Antimicrobial Properties for Simultaneous Nanoencapsulation and Extended Ocular Release Formulations of Dexamethasone and Chloramphenicol Drugs. Pharmaceutics 2020, 12, 594. [Google Scholar] [CrossRef]
- Safdar, R.; Omar, A.A.; Arunagiri, A.; Regupathi, I.; Thanabalan, M. Potential of Chitosan and its derivatives for controlled drug release applications–A review. J. Drug Deliv. Sci. Technol. 2019, 49, 642–659. [Google Scholar] [CrossRef]
- Bowman, K.; Leong, K.W. Chitosan nanoparticles for oral drug and gene delivery. Int. J. Nanomed. 2006, 1, 117–128. [Google Scholar] [CrossRef]
- Min, J.B.; Kim, E.S.; Lee, J.-S.; Lee, H.G. Preparation, characterization, and cellular uptake of resveratrol-loaded trimethyl chitosan nanoparticles. Food Sci. Biotechnol. 2017, 27, 441–450. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, F.; Aleanizy, F.; El Tahir, E.; Alhabib, H.; Alsaif, R.; Shazly, G.; AlQahtani, H.; Alsarra, I.; Mahdavi, J. Antibacterial Activity of Chitosan Nanoparticles Against Pathogenic N. gonorrhoea. Int. J. Nanomed. 2020, 15, 7877–7887. [Google Scholar] [CrossRef] [PubMed]
- Biocompatible Biodegradable Polymeric Antibacterial Nanoparticles for Enhancing the Effects of a Third-Generation Cephalosporin against Resistant Bacteria. Microbiology Society. Available online: https://www.microbiologyresearch.org/content/journal/jmm/10.1099/jmm.0.000445 (accessed on 5 December 2020).
- Furtado, G.T.F.D.S.; Fideles, T.B.; Cruz, R.D.C.A.L.; Souza, J.W.D.L.; Barbero, M.A.R.; Fook, M.V.L. Chitosan/NaF Particles Prepared Via Ionotropic Gelation: Evaluation of Particles Size and Morphology. Mater. Res. 2018, 21, 21. [Google Scholar] [CrossRef]
- Debnath, S.; Kumar, R.; Babu, M. Ionotropic gelation-A novel method to prepare chitosan nanoparticles. Res. J. Pharm. Technol. 2011, 4, 492–495. [Google Scholar]
- Saikia, C.; Gogoi, P. Chitosan: A Promising Biopolymer in Drug Delivery Applications. J. Mol. Genet. Med. 2015, 2015, 4. [Google Scholar] [CrossRef]
- Wulandari, I.O.; Mardila, V.T.; Santjojo, D.J.D.H.; Sabarudin, A. Preparation and Characterization of Chitosan-coated Fe3O4 Nanoparticles using Ex-Situ Co-Precipitation Method and Tripolyphosphate/Sulphate as Dual Crosslinkers. IOP Conf. Ser. Mater. Sci. Eng. 2018, 299, 012064. [Google Scholar] [CrossRef]
- Grenha, A. Chitosan Nanoparticles: A Survey of Preparation Methods. J. Drug Target. 2012, 20, 291–300. Available online: https://www.tandfonline.com/doi/full/10.3109/1061186X.2011.654121 (accessed on 5 December 2020). [CrossRef]
- Senthilnathan, B.; Kasiramar, G.; Vijayalakshmi, A.; Bhavya, E.; Jeyamani, s.V.; Ravichandiran, V.; Priya, S.B. Design and development of dexibuprofen loaded chitosan nanoparticles. Drug Discov. Today 2018, 10, 248. [Google Scholar]
- Nagpal, K.; Singh, S.; Mishra, D. Chitosan Nanoparticles: A Promising System in Novel Drug Delivery. Chem. Pharm. Bull. 2010, 58, 1423–1430. [Google Scholar]
- Hembram, K.C.; Prabha, S.; Chandra, R.; Ahmed, B.; Nimesh, S. Advances in preparation and characterization of chitosan nanoparticles for therapeutics. Artif. Cells Nanomed. Biotechnol. 2016, 44, 305–314. [Google Scholar] [CrossRef]
- Ibekwe, C.A.; Oyatogun, G.M.; Esan, T.A.; Oluwasegun, K.M. Synthesis and Characterization of Chitosan/Gum Arabic Nanoparticles for Bone Regeneration. Am. J. Mater. Sci. Eng. 2017, 5, 28–36. [Google Scholar]
- Sarmento, B.; Ribeiro, A.J.; Veiga, F.; Ferreira, D.C.; Neufeld, R.J. Insulin-Loaded Nanoparticles are Prepared by Alginate Ionotropic Pre-Gelation Followed by Chitosan Polyelectrolyte Complexation. J. Nanosci. Nanotechnol. 2007, 7, 2833–2841. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.; Garg, T.; Gupta, U.D.; Gupta, P.; Rath, G.; Goyal, A.K. Preparation and characterization of spray-dried inhalable powders containing nanoaggregates for pulmonary delivery of anti-tubercular drugs. Artif. Cells Nanomed. 2016, 44, 182–187. [Google Scholar] [CrossRef]
- Shakeran, Z.; Keyhanfar, M.; Varshosaz, J.; Sutherland, D.S. Biodegradable nanocarriers based on chitosan-modified mesoporous silica nanoparticles for delivery of methotrexate for application in breast cancer treatment. Mater. Sci. Eng. C 2021, 118, 111526. [Google Scholar] [CrossRef] [PubMed]
- Jafari, H.; Hassanpour, M.; Akbari, A.; Rezaie, J.; Gohari, G.; Reza Mahdavinia, G.; Jabbari, E. Characterization of pH-sensitive chitosan/hydroxypropyl methylcellulose composite nanoparticles for delivery of melatonin in cancer therapy. Mater. Lett. 2021, 282, 128818. [Google Scholar] [CrossRef]
- Matos, B.N.; Pereira, M.N.; Bravo, M.d.O.; Cunha-Filho, M.; Saldanha-Araújo, F.; Gratieri, T.; Gelfuso, G.M. Chitosan nanoparticles loading oxaliplatin as a mucoadhesive topical treatment of oral tumors: Iontophoresis further enhances drug delivery ex vivo. Int. J. Biol. Macromol. 2020, 154, 1265–1275. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Y.; Wang, L.; Liang, Z.; Li, D.; Xu, X.; Chen, Y.; Yang, X.; Zhang, H.; Niu, H. Self-crosslinkable chitosan-hyaluronic acid dialdehyde nanoparticles for CD44-targeted siRNA delivery to treat bladder cancer. Bioact. Mater. 2021, 6, 433–446. [Google Scholar] [CrossRef]
- Sohail, R.; Abbas, S.R. Evaluation of amygdalin-loaded alginate-chitosan nanoparticles as biocompatible drug delivery carriers for anticancerous efficacy. Int. J. Biol. Macromol. 2020, 153, 36–45. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Tang, X.; Huang, K.; Chen, L. Polyelectrolyte three layer nanoparticles of chitosan/dextran sulfate/chitosan for dual drug delivery. Colloids Surf. B Biointerfaces 2020, 190, 110925. [Google Scholar] [CrossRef]
- Yang, B.; Jiang, J.; Jiang, L.; Zheng, P.; Wang, F.; Zhou, Y.; Chen, Z.; Li, M.; Lian, M.; Tang, S.; et al. Chitosan mediated solid lipid nanoparticles for enhanced liver delivery of zedoary turmeric oil in vivo. Int. J. Biol. Macromol. 2020, 149, 108–115. [Google Scholar] [CrossRef]
- Khademi, Z.; Lavaee, P.; Ramezani, M.; Alibolandi, M.; Abnous, K.; Taghdisi, S.M. Co-delivery of doxorubicin and aptamer against Forkhead box M1 using chitosan-gold nanoparticles coated with nucleolin aptamer for synergistic treatment of cancer cells. Carbohydr. Polym. 2020, 248, 116735. [Google Scholar] [CrossRef] [PubMed]
- Khalil, R.M.; El Arini, S.K.; AbouSamra, M.M.; Zaki, H.S.; El-Gazaerly, O.N.; Elbary, A.A. Development of lecithin/chitosan nanoparticles for promoting topical delivery of propranolol hydrochloride: Design, optimization and in-vivo evaluation. J. Pharm. Sci. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K.M.M.S. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef] [PubMed]
- Gondil, V.S.; Dube, T.; Panda, J.J.; Yennamalli, R.M.; Harjai, K.; Chhibber, S. Comprehensive evaluation of chitosan nanoparticle based phage lysin delivery system; a novel approach to counter S. pneumoniae infections. Int. J. Pharm. 2020, 573, 118850. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, L.F.; Apolinário, A.C.; Rangel-Yagui, C.O.; Stephano, M.A.; Tavares, L.C. Chitosan nanoparticles for the delivery of a new compound active against multidrug-resistant Staphylococcus aureus. J. Drug Deliv. Sci. Technol. 2020, 55, 101363. [Google Scholar] [CrossRef]
- Akhtar, B.; Muhammad, F.; Aslam, B.; Saleemi, M.K.; Sharif, A. Pharmacokinetic profile of chitosan modified poly lactic co-glycolic acid biodegradable nanoparticles following oral delivery of gentamicin in rabbits. Int. J. Biol. Macromol. 2020, 164, 1493–1500. [Google Scholar] [CrossRef]
- Tolentino, S.; Pereira, M.N.; Cunha-Filho, M.; Gratieri, T.; Gelfuso, G.M. Targeted clindamycin delivery to pilosebaceous units by chitosan or hyaluronic acid nanoparticles for improved topical treatment of acne vulgaris. Carbohydr. Polym. 2021, 253, 117295. [Google Scholar] [CrossRef]
- Bao, Z.; Yu, A.; Shi, H.; Hu, Y.; Jin, B.; Lin, D.; Dai, M.; Lei, L.; Li, X.; Wang, Y. Glycol chitosan/oxidized hyaluronic acid hydrogel film for topical ocular delivery of dexamethasone and levofloxacin. Int. J. Biol. Macromol. 2020, 167, 659–666. [Google Scholar] [CrossRef]
- Li, J.; Jin, X.; Zhang, L.; Yang, Y.; Liu, R.; Li, Z. Comparison of Different Chitosan Lipid Nanoparticles for Improved Ophthalmic Tetrandrine Delivery: Formulation, Characterization, Pharmacokinetic and Molecular Dynamics Simulation. J. Pharm. Sci. 2020, 109, 3625–3635. [Google Scholar] [CrossRef]
- Shahab, M.S.; Rizwanullah, M.; Alshehri, S.; Imam, S.S. Optimization to development of chitosan decorated polycaprolactone nanoparticles for improved ocular delivery of dorzolamide: In vitro, ex vivo and toxicity assessments. Int. J. Biol. Macromol. 2020, 163, 2392–2404. [Google Scholar] [CrossRef]
- Takeuchi, I.; Suzuki, T.; Makino, K. Iontophoretic transdermal delivery using chitosan-coated PLGA nanoparticles for transcutaneous immunization. Colloids Surf. A Physicochem. Eng. Asp. 2021, 608, 125607. [Google Scholar] [CrossRef]
- Babii, O.; Wang, Z.; Liu, G.; Martinez, E.C.; van Drunen Littel-van den Hurk, S.; Chen, L. Low molecular weight chitosan nanoparticles for CpG oligodeoxynucleotides delivery: Impact of molecular weight, degree of deacetylation, and mannosylation on intracellular uptake and cytokine induction. Int. J. Biol. Macromol. 2020, 159, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Masjedi, M.; Azadi, A.; Heidari, R.; Mohammadi-Samani, S. Brain targeted delivery of sumatriptan succinate loaded chitosan nanoparticles: Preparation, In vitro characterization, and (Neuro-)pharmacokinetic evaluations. J. Drug Deliv. Sci. Technol. 2020, 102179, in press. [Google Scholar] [CrossRef]
- Chatzitaki, A.-T.; Jesus, S.; Karavasili, C.; Andreadis, D.; Fatouros, D.G.; Borges, O. Chitosan-coated PLGA nanoparticles for the nasal delivery of ropinirole hydrochloride: In vitro and ex vivo evaluation of efficacy and safety. Int. J. Pharm. 2020, 589, 119776. [Google Scholar] [CrossRef] [PubMed]
- Sava, V.; Fihurka, O.; Khvorova, A.; Sanchez-Ramos, J. Data on enrichment of chitosan nanoparticles for intranasal delivery of oligonucleotides to the brain. Data Brief 2020, 28, 105093. [Google Scholar] [CrossRef] [PubMed]
- Rebbouh, F.; Martin-Eauclaire, M.-F.; Laraba-Djebari, F. Chitosan nanoparticles as a delivery platform for neurotoxin II from Androctonus australis hector scorpion venom: Assessment of toxicity and immunogenicity. Acta Trop. 2020, 205, 105353. [Google Scholar]
- Wang, J.; Chin, D.; Poon, C.; Mancino, V.; Pham, J.; Li, H.; Ho, P.-Y.; Hallows, K.R.; Chung, E.J. Oral delivery of metformin by chitosan nanoparticles for polycystic kidney disease. J. Control. Release 2020. [Google Scholar] [CrossRef]
- Maria, S.; Sarwar, H.S.; Sohail, M.F.; Imran, M.; Salman Qureshi, O.; Raza, A.; Ahmad, N.M.; Iqbal, A.; Shahnaz, G. Synthesis and characterization of pre-activated thiolated chitosan nanoparticles for oral delivery of octreotide. J. Drug Deliv. Sci. Technol. 2020, 58, 101807. [Google Scholar] [CrossRef]
- Sudhakar, S.; Chandran, S.V.; Selvamurugan, N.; Nazeer, R.A. Biodistribution and pharmacokinetics of thiolated chitosan nanoparticles for oral delivery of insulin in vivo. Int. J. Biol. Macromol. 2020, 150, 281–288. [Google Scholar] [CrossRef]
- Manne, A.A.; Arigela, B.; Giduturi, A.K.; Komaravolu, R.K.; Mangamuri, U.; Poda, S. Pterocarpus marsupium Roxburgh heartwood extract/chitosan nanoparticles loaded hydrogel as an innovative wound healing agent in the diabetic rat model. Mater. Today Commun. 2021, 26, 101916. [Google Scholar] [CrossRef]
- Ataide, J.A.; Geraldes, D.C.; Gérios, E.F.; Bissaco, F.M.; Cefali, L.C.; Oliveira-Nascimento, L.; Mazzola, P.G. Freeze-dried chitosan nanoparticles to stabilize and deliver bromelain. J. Drug Deliv. Sci. Technol. 2020, 102225, in press. [Google Scholar] [CrossRef]
- Latha, S.; Selvamani, P.; Silambarasi, T.; Bibiana, A.; Prabha, T.; Mathan, G.; Thimiri Govindaraj, D.B. Development of Meloxicam-Chitosan Magnetic Nanoconjugates for targeting Rheumatoid Arthritis Joints: Pharmaceutical characterization and preclinical assessment on murine models. J. Magn. Magn. Mater. 2020, 523, 167571. [Google Scholar]
- Khan, M.A.; Chen, L.; Liang, L. Improvement in storage stability and resveratrol retention by fabrication of hollow zein-chitosan composite particles. Food Hydrocoll. 2020, 106477, in press. [Google Scholar] [CrossRef]
- Nasri, R.; Hamdi, M.; Touir, S.; Li, S.; Karra-Chaâbouni, M.; Nasri, M. Development of delivery system based on marine chitosan: Encapsulation and release kinetic study of antioxidant peptides from chitosan microparticle. Int. J. Biol. Macromol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Afshar, M.; Dini, G.; Vaezifar, S.; Mehdikhani, M.; Movahedi, B. Preparation and characterization of sodium alginate/polyvinyl alcohol hydrogel containing drug-loaded chitosan nanoparticles as a drug delivery system. J. Drug Deliv. Sci. Technol. 2020, 56, 101530. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, C.; Ding, Y.; Liu, M.; Tai, Z.; Jin, Q.; Yang, Y.; Li, Z.; Yang, M.; Gong, W.; et al. Red blood cell-hitchhiking chitosan nanoparticles for prolonged blood circulation time of vitamin K1. Int. J. Pharm. 2020, 592, 120084. [Google Scholar] [CrossRef]
- Del Prado-Audelo, M.L.; Caballero-Florán, I.H.; Sharifi-Rad, J.; Mendoza-Muñoz, N.; González-Torres, M.; Urbán-Morlán, Z.; Florán, B.; Cortes, H.; Leyva-Gómez, G. Chitosan-decorated nanoparticles for drug delivery. J. Drug Deliv. Sci. Technol. 2020, 59, 101896. [Google Scholar] [CrossRef]
- Lee, J.H.; Yeo, Y. Controlled drug release from pharmaceutical nanocarriers. Chem. Eng. Sci. 2015, 125, 75–84. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.A.; Syeda, J.T.M.; Wasan, K.M.; Wasan, E.K. An Overview of Chitosan Nanoparticles and Its Application in Non-Parenteral Drug Delivery. Pharmaceutics 2017, 9, 53. [Google Scholar] [CrossRef] [Green Version]
- Kashyap, P.L.; Xiang, X.; Heiden, P. Chitosan nanoparticle based delivery systems for sustainable agriculture. Int. J. Biol. Macromol. 2015, 77, 36–51. [Google Scholar] [CrossRef]
- Koukaras, E.N.; Papadimitriou, S.A.; Bikiaris, D.N.; Froudakis, G.E. Insight on the Formation of Chitosan Nanoparticles through Ionotropic Gelation with Tripolyphosphate. Mol. Pharm. 2012, 9, 2856–2862. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Yan, W.; Xu, Z.; Ni, H. Formation mechanism of monodisperse, low molecular weight chitosan nanoparticles by ionic gelation technique. Colloids Surf. B Biointerfaces 2012, 90, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.R.; Singh, U.S.; Momin, M.; Bhavsar, C. Chitosan: Application in tissue engineering and skin grafting. J. Polym. Res. 2017, 24, 125. [Google Scholar] [CrossRef]
- Kang, H.; Mintri, S.; Menon, A.V.; Lee, H.Y.; Choi, H.S.; Kim, J. Pharmacokinetics, pharmacodynamics and toxicology of theranostic nanoparticles. Nanoscale 2015, 7, 18848–18862. [Google Scholar] [CrossRef] [Green Version]
- El-Shabouri, M.H. Positively charged nanoparticles for improving the oral bioavailability of cyclosporin-A. Int. J. Pharm. 2002, 249, 101–108. [Google Scholar] [CrossRef]
- Xue, M.; Hu, S.; Lu, Y.; Zhang, Y.; Jiang, X.; An, S.; Guo, Y.; Zhou, X.; Hou, H.; Jiang, C. Development of chitosan nanoparticles as drug delivery system for a prototype capsid inhibitor. Int. J. Pharm. 2015, 495, 771–782. [Google Scholar] [CrossRef]
- Bagre, A.P.; Jain, K.; Jain, N.K. Alginate coated chitosan core shell nanoparticles for oral delivery of enoxaparin: In vitro and in vivo assessment. Int. J. Pharm. 2013, 456, 31–40. [Google Scholar] [CrossRef]
- Sonin, D.; Pochkaeva, E.; Zhuravskii, S.; Postnov, V.; Korolev, D.; Vasina, L.; Kostina, D.; Mukhametdinova, D.; Zelinskaya, I.; Skorik, Y.; et al. Biological Safety and Biodistribution of Chitosan Nanoparticles. Nanomaterials 2020, 10, 810. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.-L.; Qi, W.; Han, F.; Shao, J.-Z.; Gao, J.-Q. Toxicity evaluation of biodegradable chitosan nanoparticles using a zebrafish embryo model. Int. J. Nanomed. 2011, 6, 3351–3359. [Google Scholar]
- Yan, C.; Chen, D.; Gu, J.; Hu, H.; Zhao, X.; Qiao, M. Preparation of N-Succinyl-chitosan and Their Physical-Chemical Properties as a Novel Excipient. Yakugaku Zasshi 2006, 126, 789–793. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Onishi, H.; Machida, Y. Biological Fate of Highly-Succinylated N-Succinyl-chitosan and Antitumor Characteristics of Its Water-soluble Conjugate with Mitomycin C at I.v. and I.p. Administration into Tumor-Bearing Mice. Biol. Pharm. Bull. 2000, 23, 1497–1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, B.; Samanta, M.K.; Muthu, M.S.; Vinothapooshan, G. Design and evaluation of chitosan nanoparticles as novel drug carrier for the delivery of rivastigmine to treat Alzheimer’s disease. Ther. Deliv. 2011, 2, 599–609. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liu, F.; Guo, J.; Xue, J.; Qian, Z.; Gu, Y. Folate-modified chitosan micelles with enhanced tumor targeting evaluated by near infrared imaging system. Carbohydr. Polym. 2011, 86, 1118–1129. [Google Scholar] [CrossRef]
- Liu, S.; Yang, S.; Ho, P.C. Intranasal administration of carbamazepine-loaded carboxymethyl chitosan nanoparticles for drug delivery to the brain. Asian J. Pharm. Sci. 2018, 13, 72–81. [Google Scholar] [CrossRef] [PubMed]
- In Silico Analysis of Sulpiride, Synthesis, Characterization and In Vitro Studies of Its Nanoparticle for the Treatment of Schizophrenia: Ingenta Connect. Available online: https://www.ingentaconnect.com/contentone/ben/cad/2020/00000016/00000002/art00003 (accessed on 8 December 2020).
- Tzeyung, A.S.; Md, S.; Bhattamisra, S.K.; Madheswaran, T.; Alhakamy, N.A.; Aldawsari, H.M.; Radhakrishnan, A.K. Fabrication, Optimization, and Evaluation of Rotigotine-Loaded Chitosan Nanoparticles for Nose-To-Brain Delivery. Pharmaceutics 2019, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Singh, S.K.; Mishra, D.N. Nose to Brain Delivery of Galantamine Loaded Nanoparticles: In-Vivo Pharmacodynamic and Biochemical Study in Mice. Available online: https://www.eurekaselect.com/165932/article (accessed on 8 December 2020).
- Joseph, J.; Pandey, V.P. Formulation and evaluation of olanzapine loaded chitosan nanoparticles for nose to brain targeting an in vitro and ex vivo toxicity study. J. Appl. Pharm. Sci. 2016, 6, 34–40. [Google Scholar]
- Xu, J.; Xu, B.; Shou, D.; Xia, X.; Hu, Y. Preparation and Evaluation of Vancomycin-Loaded N-trimethyl Chitosan Nanoparticles. Polymers 2015, 7, 1850–1870. [Google Scholar] [CrossRef] [Green Version]
- Rossi, S.; Vigani, B.; Puccio, A.; Bonferoni, M.C.; Sandri, G.; Ferrari, F. Chitosan Ascorbate Nanoparticles for the Vaginal Delivery of Antibiotic Drugs in Atrophic Vaginitis. Mar. Drugs. 2017, 15, 319. [Google Scholar] [CrossRef] [Green Version]
- Kumar, J.; Newton, A.M.J. Rifaximin-Chitosan Nanoparticles for Inflammatory Bowel Disease (IBD). Recent Pat. Inflamm. Allergy Drug Discov. 2017, 11, 41–52. [Google Scholar] [CrossRef]
- Lee, B.-S.; Lee, C.-C.; Wang, Y.-P.; Chen, H.-J.; Lai, C.-H.; Hsieh, W.-L.; Chen, Y.-W. Controlled-release of tetracycline and lovastatin by poly(d,l-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285–297. [Google Scholar]
- Sharma, S. Chitosan Loaded Ketorolac Tromethamine Nanoparticles for Improved Ocular Delivery in Eye Inflammation. Indian J. Pharm. Educ. Res. 2018, 52, S202–S209. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tan, J.; Luo, J.; Huang, P.; Zhou, W.; Chen, L.; Long, L.; Zhang, L.I.; Zhu, B.; Yang, L.; et al. Enhancement of Scutellarin Oral Delivery Efficacy by Vitamin B12-Modified Amphiphilic Chitosan Derivatives to Treat Type II Diabetes Induced-Retinopathy. J. Nanobiotechnol. 2017, 15, 18. Available online: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-017-0251-z (accessed on 8 December 2020). [CrossRef] [PubMed] [Green Version]
- Curcumin Loaded Chitosan Nanoparticles Impregnated into Collagen-Alginate Scaffolds for Diabetic Wound Healing. Available online: https://www.researchgate.net/publication/302982389_Curcumin_loaded_chitosan_nanoparticles_impregnated_into_collagen-alginate_scaffolds_for_diabetic_wound_healing (accessed on 8 December 2020).
- Rahbarian, M.; Mortazavian, E.; Dorkoosh, F.A.; Rafiee Tehrani, M. Preparation, evaluation and optimization of nanoparticles composed of thiolated triethyl chitosan: A potential approach for buccal delivery of insulin. J. Drug Deliv. Sci. Technol. 2018, 44, 254–263. [Google Scholar] [CrossRef]
- Rong, X.; Ji, Y.; Zhu, X.; Yang, J.; Qian, D.; Mo, X.; Lu, Y. Neuroprotective Effect of Insulin-Loaded Chitosan Nanoparticles/PLGA-PEG-PLGA Hydrogel on Diabetic Retinopathy in Rats. Available online: https://www.dovepress.com/neuroprotective-effect-of-insulin-loaded-chitosan-nanoparticlesplga-pe-peer-reviewed-fulltext-article-IJN (accessed on 8 December 2020).
- Jafary Omid, N.; Bahari Javan, N.; Dehpour, A.-R.; Partoazar, A.; Rafiee Tehrani, M.; Dorkoosh, F. In-vitro and in-vivo cytotoxicity and efficacy evaluation of novel glycyl-glycine and alanyl-alanine conjugates of chitosan and trimethyl chitosan nano-particles as carriers for oral insulin delivery. Int. J. Pharm. 2018, 535, 293–307. [Google Scholar] [CrossRef]
- Hojatizade, M.; Soleymani, M.; Tafaghodi, M.; Badiee, A.; Chavoshian, O.; Jaafari, M.R. Chitosan Nanoparticles Loaded with Whole and Soluble Leishmania Antigens, and Evaluation of Their Immunogenecity in a Mouse Model of Leishmaniasis. Iran. J. Immunol. 2018, 15, 281–293. [Google Scholar]
- Dhakal, S.; Renu, S.; Ghimire, S.; Shaan Lakshmanappa, Y.; Hogshead, B.T.; Feliciano-Ruiz, N.; Lu, F.; HogenEsch, H.; Krakowka, S.; Lee, C.W.; et al. Mucosal Immunity and Protective Efficacy of Intranasal Inactivated Influenza Vaccine Is Improved by Chitosan Nanoparticle Delivery in Pigs. Front. Immunol. 2018, 9, 934. [Google Scholar] [CrossRef]
- Jesus, S.; Soares, E.; Borchard, G.; Borges, O. Poly-ϵ-caprolactone/chitosan nanoparticles provide strong adjuvant effect for hepatitis B antigen. Nanomedicine 2017, 12, 2335–2348. [Google Scholar] [CrossRef]
- Inactivated Infectious Bronchitis Virus Vaccine Encapsulated in Chitosan Nanoparticles Induces Mucosal Immune Responses and Effective Protection against Challenge-ScienceDirect. Available online: https://www.sciencedirect.com/science/article/pii/S0264410X18304341 (accessed on 8 December 2020).
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Improved In Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Xu, K.; Min, J.; Chen, M.; Shen, L.; Xu, J.; Jiang, Q.; Han, G.; Pan, L.; Li, H. Folate-conjugated hydrophobicity modified glycol chitosan nanoparticles for targeted delivery of methotrexate in rheumatoid arthritis. J. Appl. Biomater. Funct. Mater. 2020, 18, 2280800020962629. [Google Scholar] [CrossRef]
- Petkar, K.C.; Chavhan, S.; Kunda, N.; Saleem, I.; Somavarapu, S.; Taylor, K.M.G.; Sawant, K.K. Development of Novel Octanoyl Chitosan Nanoparticles for Improved Rifampicin Pulmonary Delivery: Optimization by Factorial Design. AAPS PharmSciTech 2018, 19, 1758–1772. [Google Scholar] [CrossRef]
- Hill, M.; Twigg, M.; Sheridan, E.A.; Hardy, J.G.; Elborn, J.S.; Taggart, C.C.; Scott, C.J.; Migaud, M.E. Alginate/Chitosan Particle-Based Drug Delivery Systems for Pulmonary Applications. Pharmaceutics 2019, 11, 379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buyuk, N.I.; Arayici, P.P.; Derman, S.; Mustafaeva, Z.; Yucel, Z.M.A.S. Synthesis of Chitosan Nanoparticles for Controlled Release of Amiodarone. Indian J. Pharm. Sci. 2020, 82, 131–138. [Google Scholar] [CrossRef] [Green Version]
- Jabbari, N.; Eftekhari, Z.; Roodbari, N.H.; Parivar, K. Evaluation of Encapsulated Eugenol by Chitosan Nanoparticles on the aggressive model of rheumatoid arthritis. Int. Immunopharmacol. 2020, 85, 106554. [Google Scholar] [CrossRef]
- Rawal, T.; Patel, S.; Butani, S. Chitosan nanoparticles as a promising approach for pulmonary delivery of bedaquiline. Eur. J. Pharm. Sci. 2018, 124, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Ranjbari, J.; Mokhtarzadeh, A.; Alibakhshi, A.; Tabarzad, M.; Hejazi, M.; Ramezani, M. Anti-Cancer Drug Delivery Using Carbohydrate-Based Polymers. Available online: https://www.eurekaselect.com/152236/article (accessed on 8 December 2020).
- Al-najjar, B.Y.; Hussain, S.A. chitosan microspheres for the delivery of chemotherapeutic agents: Paclitaxel as a model. Asian J. Pharm. Clin. Res. 2017, 10, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Esfandyari-Manesh, M.; Mohammadi, A.; Atyabi, F.; Nabavi, S.M.; Ebrahimi, S.M.; Shahmoradi, E.; Varnamkhasti, B.S.; Ghahremani, M.H.; Dinarvand, R. Specific targeting delivery to MUC1 overexpressing tumors by albumin-chitosan nanoparticles conjugated to DNA aptamer. Int. J. Pharm. 2016, 515, 607–615. [Google Scholar] [CrossRef]
- Choi, D.G.; Venkatesan, J.; Shim, M.S. Selective Anticancer Therapy Using Pro-Oxidant Drug-Loaded Chitosan–Fucoidan Nanoparticles. Int. J. Mol. Sci. 2019, 20, 3220. [Google Scholar] [CrossRef] [Green Version]
- Mazzotta, E.; De Benedittis, S.; Qualtieri, A.; Muzzalupo, R. Actively Targeted and Redox Responsive Delivery of Anticancer Drug by Chitosan Nanoparticles. Pharmaceutics 2020, 12, 26. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Qian, J.; Yang, M.; Xu, W.; Wang, J.; Hou, G.; Ji, L.; Suo, A. Doxorubicin/cisplatin co-loaded hyaluronic acid/chitosan-based nanoparticles for in vitro synergistic combination chemotherapy of breast cancer. Carbohydr. Polym. 2019, 225, 115206. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, W.; Lan, Y.; He, X.; Liu, K.; Liang, Y. Antitumor Effect of Hyaluronic-Acid-Modified Chitosan Nanoparticles Loaded with siRNA for Targeted Therapy for Non-Small Cell Lung Cancer. Available online: https://www.dovepress.com/antitumor-effect-of-hyaluronic-acid-modified-chitosan-nanoparticles-lo-peer-reviewed-article-IJN (accessed on 8 December 2020).
- Wang, T.; Hou, J.; Su, C.; Zhao, L.; Shi, Y. Hyaluronic Acid-Coated Chitosan Nanoparticles Induce ROS-Mediated Tumor Cell Apoptosis and Enhance Antitumor Efficiency by Targeted Drug Delivery via CD44. J. Nanobiotechnol. 2017, 15, 1–12. Available online: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/s12951-016-0245-2 (accessed on 8 December 2020). [CrossRef] [Green Version]
- Li, X.; Min, M.; Du, N.; Gu, Y.; Hode, T.; Naylor, M.; Chen, D.; Nordquist, R.E.; Chen, W.R. Chitin, Chitosan, and Glycated Chitosan Regulate Immune Responses: The Novel Adjuvants for Cancer Vaccine. Available online: https://www.hindawi.com/journals/jir/2013/387023/ (accessed on 8 December 2020).
- The Vaccine Adjuvant Chitosan Promotes Cellular Immunity via DNA Sensor cGAS-STING-Dependent Induction of Type I Interferons: Immunity. Available online: https://www.cell.com/immunity/fulltext/S1074-7613(16)30019-X?_returnURL=https%3A%2F%2Flinkinghub.elsevier.com%2Fretrieve%2Fpii%2FS107476131630019X%3Fshowall%3Dtrue (accessed on 8 December 2020).
- Tahamtan, A.; Barati, M.; Tabarraei, A.; Mohebbi, S.R.; Shirian, S.; Gorji, A.; Ghaemi, A. Antitumor Immunity Induced by Genetic Immunization with Chitosan Nanoparticle Formulated Adjuvanted for HPV-16 E7 DNA Vaccine. Iran. J. Immunol. 2018, 15, 269–280. [Google Scholar] [PubMed]
- Maiyo, F.; Singh, M. Folate-Targeted mRNA Delivery Using Chitosan-Functionalized Selenium Nanoparticles: Potential in Cancer Immunotherapy. Pharmaceuticals 2019, 12, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardo, D.; Kiselev, M.A.; Caccamo, M.T. Smart Nanoparticles for Drug Delivery Application: Development of Versatile Nanocarrier Platforms in Biotechnology and Nanomedicine. Available online: https://www.hindawi.com/journals/jnm/2019/3702518/ (accessed on 8 December 2020).
- Polysaccharide-Based Controlled Release Systems for Therapeutics Delivery and Tissue Engineering: From Bench to Bedside-Miao-2018-Advanced Science-Wiley Online Library. Available online: https://onlinelibrary.wiley.com/doi/full/10.1002/advs.201700513 (accessed on 8 December 2020).
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef] [PubMed]
- Bonferoni, M.C.; Gavini, E.; Rassu, G.; Maestri, M.; Giunchedi, P. Chitosan Nanoparticles for Therapy and Theranostics of Hepatocellular Carcinoma (HCC) and Liver-Targeting. Nanomaterials 2020, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Swierczewska, M.; Han, H.S.; Kim, K.; Park, J.H.; Lee, S. Polysaccharide-based nanoparticles for theranostic nanomedicine. Adv. Drug Deliv. Rev. 2016, 99, 70–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]





| Diseases | Drug Payloads in Chitosan NPs | Preparation Methods | Entrapment (EE) Loading Efficiency (LE) | Release Profile | Advantages of Drug Loaded Chitosan NPs | Limitations of Neat Drug | References |
|---|---|---|---|---|---|---|---|
| Breast cancer | Methotrexate-loaded chitosan-modified mesoporous silica NPs | Cross-linking of mesoporous silica NPs (modified with 3-aminopropyl triethoxysilane by glutaraldehyde | EE = 12.2% | 58.0% at pH 6.5 | 1—Amine modified NPs improved drug loading 2—Sustained drug release | 1—Unmodified MSN poor drug loading 2—Poor burst release | [25] |
| Breast cancer | Melatonin-loaded pH-sensitive chitosan/ Hydroxypropyl- methylcellulose composite NPs | Cross Linking | NA | 61% at pH 5.5 | NPs exhibited higher toxicity for MDA-MB-231 cancer cells | Low efficacy | [26] |
| Oral cancer | Oxaliplatin-loaded chitosan NPs | Iontophoresis | - | - | 1—Increased bioavailability 2—Increased drug penetration | Poor drug target specificity and penetration | [27] |
| Bladder cancer | Chitosan-hyaluronic acid dialdehyde NPs for CD44-targeted siRNA delivery | Ionotropic gelation | LE = >95% | Low cytotoxicity | Lack in target specific drug delivery | [28] | |
| Cancer | Amygdalin-loaded alginate-chitosan NPs | Polyelectrolyte complexation | EE = 90% | 86.03% at pH 7.4 after 10 h | 1—Improved cytotoxic profile 2—Sustained Drug release | Presence of cyanide group-toxicity | [29] |
| Cancer | 5-Fluorouracil-loaded chitosan/dextran sulfate/chitosan NPs for dual drug delivery | Double emulsion crosslinking method | EE: CS/DEX/CS NPs (66.3%) > CS/DEX NPs (62.4%) > CS NPs (57.3%) | 5-Fu accumulation was 99.41% at pH 5.67 after 150 h And 32% for PTX after 150 h | 1—Increased entrapment efficiency 2—controlled release 3—Enhanced inhibition in cancer cells 4—synergistic effects by dual drug delivery | - | [30] |
| Cancer | Chitosan-mediated solid lipid NPS for delivery of zedoary turmeric oil (ZTO) | Emulsion-solvent evaporation, thin film-ultrasonic dispersion | EE = 43 % | 80% after 48 h | 1—Efficient drug delivery to liver 2—Improved Bioavailability 3—Enhanced stability of ZTO | volatility, insolubility, low bioavailability | [31] |
| Cancer | Doxorubicin-aptamers-chitosan-gold NPs complex | EE = 85% | 57% after 72 h at pH 5.5 | 1—Enhanced tumor inhibitory effect 2—Less distribution to other organs | Doxorubicin alone less tumor inhibitory effect Non-specific distribution across other organs | [32] | |
| Infantile hemangioma | Propranolol hydrochloride- loaded lecithin/ chitosan NPs | double emulsion technique | EE = 53.62% | 56.11% after 24 h | 1—Minimum side effects 2—Optimum topical therapeutic concentration 3—Sustained drug release | Systemic adverse side effects | [33] |
| Antimicrobial and anticancer effects | Naringenin (NRG), quercetin (QE) and curcumin (CUR)- loaded chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide hybrid NPs | Crosslinking | LE: NRG-90.55% QE-92.84% CUR-89.89% | 70.32% (QE), 77.54% (NRG) and 65.19% (CUR) at pH 5.0 | Prominent antimicrobial activity against Staphylococcus aureus and Trichophyton rubrum strains due to synergistic effect Anticancer activity: A431 cells exhibited excellent cytotoxicity | polyphenol drugs show poor antimicrobial & anticancer activity | [34] |
| S. Pneumoniae infections | Cpl-1-loaded chitosan NPs | Ionotropic gelation method | EE = 55% | 72.6% after 24 h | 1—Increased bioavailability and half-life in-vivo 2—Chitosan NPs biocompatible candidate for Cpl-1 delivery | Low bioavailability | [35] |
| Antimicrobial activity against multidrug- resistant Staphylococcus aureus | N’-((5-nitrofuran-2-yl) methylene)-2-benzo-hydrazide was incorporated in polysorbate 20 micelles and further loaded in chitosan NPs (CH-5-NFB-NP), | Ionic gelation | EE = 44% | - | 1—Enhanced inhibition of bacterial growth 2—promising results against multi drug resistant strains 3—Easy incorporation of hydrophobic drug | 1—Drug resistance 2—Hydrophobic drug | [36] |
| Antibiotic | Gentamicin-loaded PLGA Chitosan NPs | Double emulsion method | EE = 92.5% | 74–83% after 8 h | Oral Gentamicin nano formulation showed better therapeutic effects. | No oral route due to enzymatic degradation and poor bioavailability | [37] |
| Acne vulgaris | Clindamycin-loaded chitosan NPs | Ionotropic gelation method | EE = 42% | 65% after 24 h | 1—Targeted delivery to pilosebaceous units 2—Enhanced drug distribution profile | Poor drug penetration | [38] |
| Postoperative endophthalmitis | Glycol chitosan/oxidized hyaluronic acid hydrogel film with dual release of dexamethasone (Dex) and levofloxacin (Lev) | - | - | Lev—100% release in 10 min Dex—18% in first burst release | 1—stepwise release of Lev and Dex, Lev—rapid release Dex—prolonged release 2—Potent anti-inflammatory, downregulation of inflammatory cytokines | - | [39] |
| Glaucoma, Ocular delivery | Tetrandrine lipid NPs (TET-LNPs)-loaded carboxy-methylchitosan (CMC), hydroxypropylchitosan (HPC) and trimethyl- chitosan (TMC) | Emulsion droplet method | TMC-TET-LNPs- EE% = 90.65% | 76.1% of TET after 12 h | increased ocular retention time and bioavailability | Restricted ocular bioavailability | [40] |
| Glaucoma ocular delivery | Dorzolamide-loaded chitosan decorated polycaprolactone NPs | Single step emulsification method | EE = 72.48% | 62.84% to 82.34% | 1—Improved efficacy and safety 2—Sustained release | - | [41] |
| Trans- cutaneous immunization, transdermal delivery | Poly (DL-lactide-co-glycolide) (PLGA) NPs Antigen used: hen egg-white lysozyme (HEL) | Anti-solvent diffusion method | EE = 64.3% | NA | Iontophoresis and NP efficiently delivered HEL Antibody titers in greater concentration | Subcutaneous injection of HEL solution showed low accumulation in hair follicles | [42] |
| Immuno- therapeutic agents | Low molecular weight chitosan NPs for CpG oligodeoxy- nucleotide delivery | Ionic gelation method | EE = 88.09% to 97.34% | NA | 1—Improved binding ability and intracellular uptake 2—Efficient immunostimulatory effect | Effective intracellular delivery is challenging | [43] |
| Migraine, intranasal & intravenous | Sumatriptan succinate-loaded chitosan NPs | Ionic gelation technique | EE = 59.60% | 58% after 24 h | Target specific drug delivery | limited systemic availability of sumatriptan via oral administration | [44] |
| Parkinson, nasal delivery | Ropinirole Hydrochloride-loaded chitosan-coated PLGA NPs | Nano-precipitation method | LE = 5.7% | 89% | Intranasal delivery surpasses hepatic metabolism and passes BBB to deliver Ropinirole. | 1—High first pass metabolism 2—lower bioavailability | [45] |
| Gene therapy, intranasal delivery | siRNA-loaded chitosan NPs | Polyelectrolyte complexation method | - | - | 1—Enhanced stability 2—Noninvasive gene therapy | Low stability | [46] |
| Scorpion Envenoming therapy, vaccine delivery | Chitosan NP encapsulating Aah II toxin | Ionotropic gelation | EE = 96.66% | Burst effect release of 55.37% in 8 h & 80.16% after 120 h | 1—Enhanced immunization 2—Elicitation of systemic innate and humoral immune responses | - | [47] |
| Polycystic kidney disease, Oral delivery | Metformin-loaded chitosan NPs | Ionotropic gelation method | LE = 32.2% | >50% at pH 6.5 | 1—Reduced off target side effects 2—Improved Therapeutic efficacy 3—Increased oral bioavailability | Poor bioavailability | [48] |
| Peptide Macromolecule delivery via oral route | Octreotide-loaded pre-activated thiolated chitosan NPs | Ionic gelation | EE% = 85% to 91% | TCSNPs shows 50% after 6 h | 1—Pre-activated thiomers prevent the oxidation of -SH group 2—Controlled drug release | Oxidation of -SH group, decreased efficacy | [49] |
| Diabetes, oral delivery | Insulin-loaded thiolated chitosan NPs | Microemulsion method | EE = 79.63% at pH 5.3 | 92% (after 24 h) at pH 5.3 | 1—Oral delivery-better patient compliance 2—Increased bioavailability due to interaction with the mucosal membrane of intestine 3—Prolonged drug delivery | Poor patient compliance due to subcutaneous route of injection | [50] |
| Diabetic wound healing | Pterocarpus marsupium heartwood extract/chitosan NPs (PMCNPs)-loaded hydrogel (PM-CNPsH) | Ionic gelation | - | 44% in 17 h of PM-CNPsH and 38% in 15 h | 1—Quicker wound healing in diabetes 2—Sustained Release | - | [51] |
| Wound healing and improvement in blood circulation | Chitosan-bromelain NPs | Ionic crosslinking | EE = 85.1% | - | 1—Reduced the degradation caused by protease immobilization 2—Freeze-dried-improved stability | 1—Unstable, suffers autolysis 2—Unstable when stored as aqueous suspension | [52] |
| Rheumatoid arthritis | Meloxicam-loaded chitosan-magnetite nanoconjugates | Co-precipitation | EE = 82% | 98% after 6 h | Enhanced regional bioavailability, reducing dose frequency and dose related toxicity | Low bioavailability | [53] |
| Antioxidant | Resveratrol-loaded zein chitosan NPs | - | EE = 91% | - | 1—Improved storage stability of Resveratrol 2—Sustained release | Poor stability on storage | [54] |
| Antioxidant peptides administration | Goby fish protein hydrolysate encapsulated in blue crab chitosan | Ionic gelation | EE = 58% | 60.84% | 1—Enhanced thermal stability 2—Diffusion-controlled mechanism 3—Improved antioxidant activity | Poor thermal stability | [55] |
| Hyper-lipidemia | Sodium alginate/polyvinyl alcohol hydrogel containing rosuvastatin-loaded chitosan NPs | Ionic gelation method | - | 67% after 8 h and then slow release of only 20% between 8–24 h | 1—Optimal mechanical properties 2—Controlled drug release | - | [56] |
| Prolonged blood circulation time of vitamin K1 | Chitosan NPs loading VK1 adsorbed onto red blood cells | Ionotropic gelation | EE = 78.17% | 80% after 10 days | 1—Sustained release and prolonged circulation time of vitamin K1 2—Circulation time of RBC-hitchhiking chitosan NPs greater than regular NPs | Rapid clearance from circulation by mononuclear phagocyte system | [57] |
| Swelling | Diffusion | Erosion |
|---|---|---|
| Drug release is controlled by degree of swelling. | Drug release is controlled by diffusion down the concentration gradient. | Drug release is controlled by physical or chemical degradation(erosion) of a polymer drug delivery system. |
| Water imbibition allows the drug to diffuse out. | This is based on Fickian model. | This can be due to surface or bulk erosion. |
| This can be linear for a short duration at interface. | This can be matrix-based or reservoir-based. | This depends on the surrounding medium or based on the presence of enzymes. |
| Diseases | Drug in Chitosan NPs | Zeta Potential & Loading Efficiency | References |
|---|---|---|---|
| Epilepsy | Carboxymethyl chitosan NPs as a carrier to deliver carbamazepine (CBZ-NPs) | Zeta potential of −32.1 with EE of 81.92% | [75] |
| Schizophrenia | Sulpiride-loaded chitosan NPs | EE and LE of 92.8% and 28% respectively | [76] |
| Parkinson’s disease | Rotigotine-loaded Chitosan NPs | Zeta potential (25.53 mV), and EE (96.08%) | [77] |
| Alzheimer | Galantamine-loaded thiolated chitosan NPs | - | [78] |
| Antipsychotic | Olanzapine-loaded chitosan NPs | The EE and LE was found to be 72.42% and 26.04 | [79] |
| Diseases | Drug in Chitosan NPs | Zeta Potential & Loading Efficiency | References |
|---|---|---|---|
| Chronic Osteomyelitis | Vancomycin-loaded N-trimethylchitosan NPs | Zeta potential of 14.6 mV & LE of 73.65% | [80] |
| Atrophic Vaginitis | Chitosan ascorbate NPs loaded with amoxicillin trihydrate | - | [81] |
| Inflammatory Bowel Disease | Rifaximin-chitosan NPs | Zeta potential of 37.79, EE of 73% | [82] |
| Periodontitis | Tetracycline and lovastatin by poly (d,l-lactide-co-glycolide acid)-chitosan NPs | - | [83] |
| Post-operative eye inflammation (after cataract surgery) and allergic conjunctivitis | Chitosan-loaded ketorolac tromethamine NPs | Zeta potential of −21.8 & EE of 61.65% | [84] |
| Diseases | Drug in Chitosan NPS | Zeta Potential & Loading Efficiency | References |
|---|---|---|---|
| Diabetes induced retinopathy | Scutellarin-loaded amphiphilic chitosan derivatives (Chit-DC-VB12) | Zeta potential of 16.5 mV & LE of 13% | [85] |
| Diabetic wound healing | Curcumin-loaded chitosan NPs impregnated into collagen-alginate scaffolds | Zeta potential of 30.3 mV | [86] |
| Diabetes | Insulin-loaded thiolated N-triethyl chitosan NPs | Zeta potential of 24.6 mV & EE of 97.8% | [87] |
| Diseases | Drugs in Chitosan NPs | References |
|---|---|---|
| Leishmaniasis | Chitosan NPs loaded with whole and soluble Leishmania antigens | [90] |
| Influenza | Killed SwIAV H1N2 (δ-lineage) antigens (KAg) encapsulated in chitosan NPs | [91] |
| Hepatitis B | Poly-ϵ-caprolactone/chitosan NPs provide strong adjuvant effect for hepatitis B antigen | [92] |
| Diseases | Drug in Chitosan NPs | Zeta Potential & Loading Efficiency | References |
|---|---|---|---|
| Hypertension | ACE-inhibitory biopeptides encapsulated in chitosan NPs | Zeta potential of 48.78 mV & EE of 75.36% | [94] |
| Rheumatoid arthritis | Folic acid (FA) conjugated glycolchitosan (GC) NPs (FA-GC-SA) encapsulating MTX (methotrexate) | - | [95] |
| Tuberculosis | Rifampicin-loaded octanoyl chitosan NPs | EE of 64.86% | [96] |
| Cystic Fibrosis | Tobramycin-loaded alginate/chitosan NPs | Zeta potential of 21.6 mV & EE of 44.5 | [97] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jhaveri, J.; Raichura, Z.; Khan, T.; Momin, M.; Omri, A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules 2021, 26, 272. https://doi.org/10.3390/molecules26020272
Jhaveri J, Raichura Z, Khan T, Momin M, Omri A. Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules. 2021; 26(2):272. https://doi.org/10.3390/molecules26020272
Chicago/Turabian StyleJhaveri, Jhanvi, Zarna Raichura, Tabassum Khan, Munira Momin, and Abdelwahab Omri. 2021. "Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics" Molecules 26, no. 2: 272. https://doi.org/10.3390/molecules26020272
APA StyleJhaveri, J., Raichura, Z., Khan, T., Momin, M., & Omri, A. (2021). Chitosan Nanoparticles-Insight into Properties, Functionalization and Applications in Drug Delivery and Theranostics. Molecules, 26(2), 272. https://doi.org/10.3390/molecules26020272