Nanostructure Engineering of Metal–Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst
Abstract
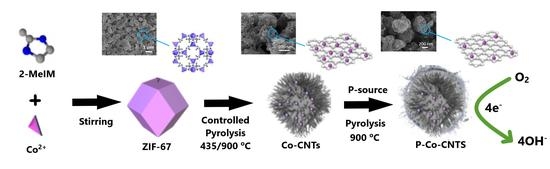
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.2.1. Synthesis of ZIF-67
3.2.2. Synthesis of Co-CN
3.2.3. Synthesis of Co-CNTs
3.2.4. Synthesis of P-Co-CNTs
3.3. Physicochemical Characterization
3.4. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Shah, S.S.A.; Najam, T.; Wen, M.; Zang, S.-Q.; Waseem, A.; Jiang, H.-L. Metal-Organic Framework-based Electrocatalysts for CO2 Reduction. Small Struct. 2021, 2100090. [Google Scholar] [CrossRef]
- Gawande, M.B.; Goswami, A.; Asefa, T.; Guo, H.; Biradar, A.V.; Peng, D.-L.; Zboril, R.; Varma, R.S. Core-shell nanoparticles: Synthesis and applications in catalysis and electrocatalysis. Chem. Soc. Rev. 2015, 44, 7540–7590. [Google Scholar] [CrossRef] [PubMed]
- Jing, S.; Zhang, L.; Luo, L.; Lu, J.; Yin, S.; Shen, P.K.; Tsiakaras, P. N-Doped Porous Molybdenum Carbide Nanobelts as Efficient Catalysts for Hydrogen Evolution Reaction. Appl. Catal. B Environ. 2018, 224, 533–540. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Jaroniec, M.; Qiao, S.Z. Heteroatom-Doped Graphene-Based Materials for Energy-Relevant Electrocatalytic Processes. ACS Catal. 2015, 5, 5207–5234. [Google Scholar] [CrossRef]
- Tzorbatzoglou, F.; Brouzgou, A.; Tsiakaras, P. Electrocatalytic activity of Vulcan-XC-72 supported Pd, Rh and PdxRhy toward HOR and ORR. Appl. Catal. B Environ. 2015, 174–175, 203–211. [Google Scholar] [CrossRef]
- Najam, T.; Ahmad Shah, S.S.; Ali, H.; Song, Z.; Sun, H.; Peng, Z.; Cai, X. A metal free electrocatalyst for high-performance zinc-air battery application with good resistance towards poisoning species. Carbon 2020, 164, 12–18. [Google Scholar] [CrossRef]
- Colic, V.; Bandarenka, A.S. Pt Alloy Electrocatalysts for the Oxygen Reduction Reaction: From Model Surfaces to Nanostructured Systems. ACS Catal. 2016, 6, 5378–5385. [Google Scholar] [CrossRef]
- Wu, R.; Tsiakaras, P.; Shen, P.K. Facile synthesis of bimetallic Pt-Pd symmetry-broken concave nanocubes and their enhanced activity toward oxygen reduction reaction. Appl. Catal. B Environ. 2019, 251, 49–56. [Google Scholar] [CrossRef]
- Long, G.-F.; Li, X.-H.; Wan, K.; Liang, Z.-X.; Piao, J.-H.; Tsiakaras, P. Pt/CN-doped electrocatalysts: Superior electrocatalytic activity for methanol oxidation reaction and mechanistic insight into interfacial enhancement. Appl. Catal. B Environ. 2017, 203, 541–548. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Aslam, M.K.; Ashfaq, M.; Rahman, M.M.; Wang, K.; Tsiakaras, P.; Song, S.; Wang, Y. Recent advances on oxygen reduction electrocatalysis: Correlating the characteristic properties of metal organic frameworks and the derived nanomaterials. Appl. Catal. B Environ. 2020, 268, 118570. [Google Scholar] [CrossRef]
- Li, Y.; Karimi, M.; Gong, Y.-N.; Dai, N.; Safarifard, V.; Jiang, H.-L. Integration of metal-organic frameworks and covalent organic frameworks: Design, synthesis, and applications. Matter 2021, 4, 2230–2265. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Rahman, M.M. Metal-organic framework-derived catalysts for Zn-Air batteries. In Handbook of Nanomaterials and Nanocomposites for Energy and Environmental Applications; Kharissova, O.V., Martínez, L.M.T., Kharisov, B.I., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–15. [Google Scholar]
- Aslam, M.K.; Shah, S.S.A.; Li, S.; Chen, C. Kinetically controlled synthesis of MOF nanostructures: Single-holed hollow core–shell ZnCoS@Co9S8/NC for ultra-high performance lithium-ion batteries. J. Mater. Chem. A 2018, 6, 14083–14090. [Google Scholar] [CrossRef]
- Zhang, W.; Cai, G.; Wu, R.; He, Z.; Yao, H.-B.; Jiang, H.-L.; Yu, S.-H. Templating Synthesis of Metal–Organic Framework Nanofiber Aerogels and Their Derived Hollow Porous Carbon Nanofibers for Energy Storage and Conversion. Small 2021, 2004140. [Google Scholar] [CrossRef]
- Sun, K.; Liu, M.; Pei, J.; Li, D.; Ding, C.; Wu, K.; Jiang, H.-L. Incorporating Transition-Metal Phosphides into Metal-Organic Frameworks for Enhanced Photocatalysis. Angew. Chem. Int. Ed. 2020, 59, 22749–22755. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.S.A.; Najam, T.; Javed, M.S.; Rahman, M.M.; Tsiakaras, P. Novel Mn-/Co-Nx Moieties Captured in N-Doped Carbon Nanotubes for Enhanced Oxygen Reduction Activity and Stability in Acidic and Alkaline Media. ACS Appl. Mater. Interfaces 2021, 13, 23191–23200. [Google Scholar] [CrossRef] [PubMed]
- Xia, W.; Mahmood, A.; Zou, R.; Xu, Q. Metal-organic frameworks and their derived nanostructures for electrochemical energy storage and conversion. Energy Environ. Sci. 2015, 8, 1837–1866. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.S.A.; Ding, W.; Wei, Z. Role of P-doping in Antipoisoning: Efficient MOF-Derived 3D Hierarchical Architectures for the Oxygen Reduction Reaction. J. Phys. Chem. C 2019, 123, 16796–16803. [Google Scholar] [CrossRef]
- Chen, Y.-Z.; Wang, C.; Wu, Z.-Y.; Xiong, Y.; Xu, Q.; Yu, S.-H.; Jiang, H.-L. From Bimetallic Metal-Organic Framework to Porous Carbon: High Surface Area and Multicomponent Active Dopants for Excellent Electrocatalysis. Adv. Mater. 2015, 27, 5010–5016. [Google Scholar] [CrossRef] [PubMed]
- You, B.; Jiang, N.; Sheng, M.; Drisdell, W.S.; Yano, J.; Sun, Y. Bimetal–Organic Framework Self-Adjusted Synthesis of Support-Free Nonprecious Electrocatalysts for Efficient Oxygen Reduction. ACS Catal. 2015, 5, 7068–7076. [Google Scholar] [CrossRef] [Green Version]
- Shah, S.S.A.; Peng, L.S.; Najam, T.; Cheng, C.; Wu, G.P.; Nie, Y.; Ding, W.; Qi, X.Q.; Chen, S.G.; Wei, Z.D. Monodispersed Co in Mesoporous Polyhedrons: Fine-tuning of ZIF-8 Structure with Enhanced Oxygen Reduction Activity. Electrochim. Acta 2017, 251, 498–504. [Google Scholar] [CrossRef]
- Wu, X.; Meng, G.; Liu, W.; Li, T.; Yang, Q.; Sun, X.; Liu, J. Metal-organic framework-derived, Zn-doped porous carbon polyhedra with enhanced activity as bifunctional catalysts for rechargeable zinc-air batteries. Nano Res. 2018, 11, 163–173. [Google Scholar] [CrossRef]
- Indra, A.; Song, T.; Paik, U. Metal Organic Framework Derived Materials: Progress and Prospects for the Energy Conversion and Storage. Adv. Mater. 2018, 30, 1705146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Wang, R.; Hong, M. Structural Evolution from Metal–Organic Framework to Hybrids of Nitrogen-Doped Porous Carbon and Carbon Nanotubes for Enhanced Oxygen Reduction Activity. Chem. Mater. 2015, 27, 7610–7618. [Google Scholar] [CrossRef]
- Pachfule, P.; Shinde, D.; Majumder, M.; Xu, Q. Fabrication of carbon nanorods and graphene nanoribbons from a metal–organic framework. Nat. Chem. 2016, 8, 718–724. [Google Scholar] [CrossRef]
- Lee, H.J.; Cho, W.; Lim, E.; Oh, M. One-pot synthesis of magnetic particle-embedded porous carbon composites from metal-organic frameworks and their sorption properties. Chem. Commun. 2014, 50, 5476–5479. [Google Scholar] [CrossRef]
- Zheng, F.; Xia, G.; Yang, Y.; Chen, Q. MOF-derived ultrafine MnO nanocrystals embedded in a porous carbon matrix as high-performance anodes for lithium-ion batteries. Nanoscale 2015, 7, 9637–9645. [Google Scholar] [CrossRef]
- Shah, S.S.A.; Najam, T.; Cheng, C.; Peng, L.; Xiang, R.; Zhang, L.; Deng, J.; Ding, W.; Wei, Z. Exploring Fe-Nx for Peroxide Reduction: Template-Free Synthesis of Fe-Nx Traumatized Mesoporous Carbon Nanotubes as an ORR Catalyst in Acidic and Alkaline Solutions. Chem. Eur. J. 2018, 24, 10630–10635. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Z.-Y.; Jiang, H.-L.; Yu, S.-H. Nanowire-Directed Templating Synthesis of Metal–Organic Framework Nanofibers and Their Derived Porous Doped Carbon Nanofibers for Enhanced Electrocatalysis. J. Am. Chem. Soc. 2014, 136, 14385–14388. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhao, P.; Hua, X.; Luo, W.; Chen, S.; Cheng, G. An Fe-N-C hybrid electrocatalyst derived from a bimetal-organic framework for efficient oxygen reduction. J. Mater. Chem. A 2016, 4, 11357–11364. [Google Scholar] [CrossRef]
- Chen, L.; Bai, J.; Wang, C.; Pan, Y.; Scheer, M.; You, X. One-step solid-state thermolysis of a metal-organic framework: A simple and facile route to large-scale of multiwalled carbon nanotubes. Chem. Commun. 2008, 13, 1581–1583. [Google Scholar] [CrossRef] [PubMed]
- Barman, B.K.; Nanda, K.K. Prussian blue as a single precursor for synthesis of Fe/Fe3C encapsulated N-doped graphitic nanostructures as bi-functional catalysts. Green Chem. 2016, 18, 427–432. [Google Scholar] [CrossRef]
- Li, D.; Senevirathne, K.; Aquilina, L.; Brock, S.L. Effect of Synthetic Levers on Nickel Phosphide Nanoparticle Formation: Ni5P4 and NiP2. Inorg. Chem. 2015, 54, 7968–7975. [Google Scholar] [CrossRef] [PubMed]
- Habas, S.E.; Baddour, F.G.; Ruddy, D.A.; Nash, C.P.; Wang, J.; Pan, M.; Hensley, J.E.; Schaidle, J.A. A Facile Molecular Precursor Route to Metal Phosphide Nanoparticles and Their Evaluation as Hydrodeoxygenation Catalysts. Chem. Mater. 2015, 27, 7580–7592. [Google Scholar] [CrossRef]
- Zhong, X.; Jiang, Y.; Chen, X.; Wang, L.; Zhuang, G.; Li, X.; Wang, J.-G. Integrating cobalt phosphide and cobalt nitride-embedded nitrogen-rich nanocarbons: High-performance bifunctional electrocatalysts for oxygen reduction and evolution. J. Mater. Chem. A 2016, 4, 10575–10584. [Google Scholar] [CrossRef]
- Najam, T.; Ahmad Shah, S.S.; Ding, W.; Ling, Z.; Li, L.; Wei, Z. Electron penetration from metal core to metal species attached skin in nitrogen-doped core-shell catalyst for enhancing oxygen evolution reaction. Electrochim. Acta 2019, 327, 134939. [Google Scholar] [CrossRef]
- Gong, K.; Du, F.; Xia, Z.; Durstock, M.; Dai, L. Nitrogen-Doped Carbon Nanotube Arrays with High Electrocatalytic Activity for Oxygen Reduction. Science 2009, 323, 760–764. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Jiao, Y.; Jaroniec, M.; Jin, Y.; Qiao, S.Z. Nanostructured Metal-Free Electrochemical Catalysts for Highly Efficient Oxygen Reduction. Small 2012, 8, 3550–3566. [Google Scholar] [CrossRef]
- Yu, D.; Nagelli, E.; Du, F.; Dai, L. Metal-Free Carbon Nanomaterials Become More Active than Metal Catalysts and Last Longer. J. Phys. Chem. Lett. 2010, 1, 2165–2173. [Google Scholar] [CrossRef]
- Hu, X.; Wu, Y.; Li, H.; Zhang, Z. Adsorption and Activation of O2 on Nitrogen-Doped Carbon Nanotubes. J. Phys. Chem. C. 2010, 114, 9603–9607. [Google Scholar] [CrossRef]
- Najam, T.; Shah, S.S.A.; Ding, W.; Jiang, J.; Jia, L.; Yao, W.; Li, L.; Wei, Z. An Efficient Anti-poisoning Catalyst against SOx, NOx, and POx: P, N-Doped Carbon for Oxygen Reduction in Acidic Media. Angew. Chem. Int. Ed. 2018, 57, 15101–15106. [Google Scholar] [CrossRef] [PubMed]
- Elmas, S.; Beelders, W.; Pan, X.; Nann, T. Conducting Copper (I/II)-Metallopolymer for the Electrocatalytic Oxygen Reduction Reaction (ORR) with High Kinetic Current Density. Polymers 2018, 10, 1002. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, S.S.A.; Najam, T.; Molochas, C.; Nazir, M.A.; Brouzgou, A.; Javed, M.S.; Rehman, A.u.; Tsiakaras, P. Nanostructure Engineering of Metal–Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst. Molecules 2021, 26, 6672. https://doi.org/10.3390/molecules26216672
Shah SSA, Najam T, Molochas C, Nazir MA, Brouzgou A, Javed MS, Rehman Au, Tsiakaras P. Nanostructure Engineering of Metal–Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst. Molecules. 2021; 26(21):6672. https://doi.org/10.3390/molecules26216672
Chicago/Turabian StyleShah, Syed Shoaib Ahmad, Tayyaba Najam, Costas Molochas, Muhammad Altaf Nazir, Angeliki Brouzgou, Muhammad Sufyan Javed, Aziz ur Rehman, and Panagiotis Tsiakaras. 2021. "Nanostructure Engineering of Metal–Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst" Molecules 26, no. 21: 6672. https://doi.org/10.3390/molecules26216672
APA StyleShah, S. S. A., Najam, T., Molochas, C., Nazir, M. A., Brouzgou, A., Javed, M. S., Rehman, A. u., & Tsiakaras, P. (2021). Nanostructure Engineering of Metal–Organic Derived Frameworks: Cobalt Phosphide Embedded in Carbon Nanotubes as an Efficient ORR Catalyst. Molecules, 26(21), 6672. https://doi.org/10.3390/molecules26216672