A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics
Abstract
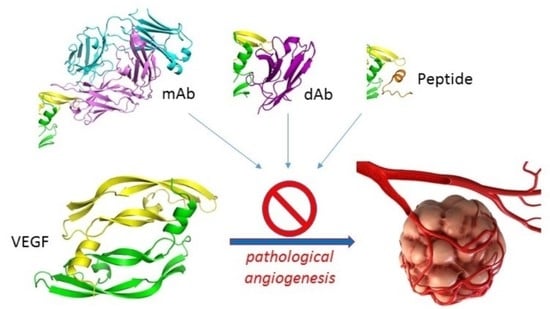
:1. Introduction
1.1. Vasculogenesis, Angiogenesis, and Lymphangiogenesis
1.2. The VEGF Family of Growth Factors
1.3. The VEGF Receptors and Co-Receptors
1.4. Scope of the Review
2. Ligands of VEGF-A
2.1. Receptors and Receptor Fragments
2.2. Antibodies and Antibody Fragments
2.3. Aptamers
2.4. Small Proteins and Peptides

2.5. Small Molecules
2.6. Analysis of the Binding Modes of VEGF-A Ligands
3. Ligands of PlGF and VEGF-B
3.1. Receptors and Receptor Fragments
3.2. Antibodies and Antibody Fragments
3.3. Small Proteins and Peptides
4. Ligands of VEGF-C and VEGF-D
4.1. Receptors and Receptor Fragments
4.2. Antibodies
5. Ligands of Non-Mammalian VEGFs
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Risau, W.; Flamme, I. Vasculogenesis. Annu. Rev. Cell Dev. Biol. 1995, 11, 73–91. [Google Scholar] [CrossRef]
- Risau, W. Mechanisms of angiogenesis. Nature 1997, 386, 671–674. [Google Scholar] [CrossRef]
- Carmeliet, P.; Ng, Y.S.; Nuyens, D.; Theilmeier, G.; Brusselmans, K.; Cornelissen, I.; Ehler, E.; Kakkar, V.V.; Stalmans, I.; Mattot, V.; et al. Impaired myocardial angiogenesis and ischemic cardiomyopathy in mice lacking the vascular endothelial growth factor isoforms VEGF164 and VEGF188. Nat. Med. 1999, 5, 495–502. [Google Scholar] [CrossRef]
- Keyt, B.A.; Nguyen, H.V.; Berleau, L.T.; Duarte, C.M.; Park, J.; Chen, H.; Ferrara, N. Identification of vascular endothelial growth factor determinants for binding KDR and FLT-1 receptors. Generation of receptor-selective VEGF variants by site-directed mutagenesis. J. Biol. Chem. 1996, 271, 5638–5646. [Google Scholar] [CrossRef] [Green Version]
- Melincovici, C.S.; Bosca, A.B.; Susman, S.; Marginean, M.; Mihu, C.; Istrate, M.; Moldovan, I.M.; Roman, A.L.; Mihu, C.M. Vascular endothelial growth factor (VEGF)—Key factor in normal and pathological angiogenesis. Rom. J. Morphol. Embryol. 2018, 59, 455–467. [Google Scholar]
- Achen, M.G.; McColl, B.K.; Stacker, S.A. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell 2005, 7, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Alitalo, K.; Tammela, T.; Petrova, T.V. Lymphangiogenesis in development and human disease. Nature 2005, 438, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Tobler, N.E.; Detmar, M. Tumor and lymph node lymphangiogenesis—Impact on cancer metastasis. J. Leukoc. Biol. 2006, 80, 691–696. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warren, A.G.; Brorson, H.; Borud, L.J.; Slavin, S.A. Lymphedema: A comprehensive review. Ann. Plast. Surg. 2007, 59, 464–472. [Google Scholar] [CrossRef]
- Tammela, T.; Alitalo, K. Lymphangiogenesis: Molecular mechanisms and future promise. Cell 2010, 140, 460–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, A.S.; Ferrara, N. Developmental and pathological angiogenesis. Annu. Rev. Cell Dev. Biol. 2011, 27, 563–584. [Google Scholar] [CrossRef]
- Maby-El Hajjami, H.; Petrova, T.V. Developmental and pathological lymphangiogenesis: From models to human disease. Histochem. Cell Biol. 2008, 130, 1063–1078. [Google Scholar] [CrossRef] [Green Version]
- Iyer, S.; Acharya, K.R. Tying the knot: The cystine signature and molecular-recognition processes of the vascular endothelial growth factor family of angiogenic cytokines. FEBS J. 2011, 278, 4304–4322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N. VEGF and intraocular neovascularization: From discovery to therapy. Transl. Vis. Sci. Technol. 2016, 5, 10. [Google Scholar] [CrossRef] [Green Version]
- Suto, K.; Yamazaki, Y.; Morita, T.; Mizuno, H. Crystal structures of novel vascular endothelial growth factors (VEGF) from snake venoms: Insight into selective VEGF binding to kinase insert domain-containing receptor but not to fms-like tyrosine kinase-1. J. Biol. Chem. 2005, 280, 2126–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieren, M.; Prota, A.E.; Ruch, C.; Kostrewa, D.; Wagner, A.; Biedermann, K.; Winkler, F.K.; Ballmer-Hofer, K. Crystal structure of the Orf virus NZ2 variant of vascular endothelial growth factor-E. Implications for receptor specificity. J. Biol. Chem. 2006, 281, 19578–19587. [Google Scholar] [CrossRef] [Green Version]
- Lyttle, D.J.; Fraser, K.M.; Fleming, S.B.; Mercer, A.A.; Robinson, A.J. Homologs of vascular endothelial growth factor are encoded by the poxvirus orf virus. J. Virol. 1994, 68, 84–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, Y.; Morita, T. Molecular and functional diversity of vascular endothelial growth factors. Mol. Divers. 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, N.; Henzel, W.J. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem. Biophys. Res. Commun. 1989, 161, 851–858. [Google Scholar] [CrossRef]
- Poltorak, Z.; Cohen, T.; Sivan, R.; Kandelis, Y.; Spira, G.; Vlodavsky, I.; Keshet, E.; Neufeld, G. VEGF145, a secreted vascular endothelial growth factor isoform that binds to extracellular matrix. J. Biol. Chem. 1997, 272, 7151–7158. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Jiang, A.; Pei, D. Identification and characterization of a new splicing variant of vascular endothelial growth factor: VEGF183. Biochim. Biophys. Acta. 1998, 1443, 400–406. [Google Scholar] [CrossRef]
- Ferrara, N. Binding to the extracellular matrix and proteolytic processing: Two key mechanisms regulating vascular endothelial growth factor action. Mol. Biol. Cell 2010, 21, 687–690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houck, K.A.; Leung, D.W.; Rowland, A.M.; Winer, J.; Ferrara, N. Dual regulation of vascular endothelial growth factor bioavailability by genetic and proteolytic mechanisms. J. Biol. Chem. 1992, 267, 26031–26037. [Google Scholar] [CrossRef]
- Maglione, D.; Guerriero, V.; Viglietto, G.; Delli-Bovi, P.; Persico, M.G. Isolation of a human placenta cDNA coding for a protein related to the vascular permeability factor. Proc. Natl. Acad. Sci. USA 1991, 88, 9267–9271. [Google Scholar] [CrossRef] [Green Version]
- Olofsson, B.; Pajusola, K.; Kaipainen, A.; von Euler, G.; Joukov, V.; Saksela, O.; Orpana, A.; Pettersson, R.F.; Alitalo, K.; Eriksson, U. Vascular endothelial growth factor B, a novel growth factor for endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 2576–2581. [Google Scholar] [CrossRef] [Green Version]
- Joukov, V.; Pajusola, K.; Kaipainen, A.; Chilov, D.; Lahtinen, I.; Kukk, E.; Saksela, O.; Kalkkinen, N.; Alitalo, K. A novel vascular endothelial growth factor, VEGF-C, is a ligand for the Flt4 (VEGFR-3) and KDR (VEGFR-2) receptor tyrosine kinases. EMBO J. 1996, 15, 290–298. [Google Scholar] [CrossRef]
- Achen, M.G.; Jeltsch, M.; Kukk, E.; Makinen, T.; Vitali, A.; Wilks, A.F.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor D (VEGF-D) is a ligand for the tyrosine kinases VEGF receptor 2 (Flk1) and VEGF receptor 3 (Flt4). Proc. Natl. Acad. Sci. USA 1998, 95, 548–553. [Google Scholar] [CrossRef] [Green Version]
- Hauser, S.; Weich, H.A. A heparin-binding form of placenta growth factor (PlGF-2) is expressed in human umbilical vein endothelial cells and in placenta. Growth Factors 1993, 9, 259–268. [Google Scholar] [CrossRef]
- Olofsson, B.; Pajusola, K.; von Euler, G.; Chilov, D.; Alitalo, K.; Eriksson, U. Genomic organization of the mouse and human genes for vascular endothelial growth factor B (VEGF-B) and characterization of a second splice isoform. J. Biol. Chem. 1996, 271, 19310–19317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Ahn, H.; Hinrichs, M.; Torry, R.J.; Torry, D.S. Evidence of a novel isoform of placenta growth factor (PlGF-4) expressed in human trophoblast and endothelial cells. J. Reprod. Immunol. 2003, 60, 53–60. [Google Scholar] [CrossRef]
- Cao, Y.; Ji, W.R.; Qi, P.; Rosin, A.; Cao, Y. Placenta growth factor: Identification and characterization of a novel isoform generated by RNA alternative splicing. Biochem. Biophys. Res. Commun. 1997, 235, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Scotney, P.D.; Nash, A.D.; Ravi Acharya, K. Crystal structure of human vascular endothelial growth factor-B: Identification of amino acids important for receptor binding. J. Mol. Biol. 2006, 359, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Lohela, M.; Bry, M.; Tammela, T.; Alitalo, K. VEGFs and receptors involved in angiogenesis versus lymphangiogenesis. Curr. Opin. Cell. Biol. 2009, 21, 154–165. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Chen, H.; Zhou, L.; Chiang, M.K.; Anand-Apte, B.; Weatherbee, J.A.; Wang, Y.; Fang, F.; Flanagan, J.G.; Tsang, M.L. Heterodimers of placenta growth factor/vascular endothelial growth factor. Endothelial activity, tumor cell expression, and high affinity binding to Flk-1/KDR. J. Biol. Chem. 1996, 271, 3154–3162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domigan, C.K.; Ziyad, S.; Iruela-Arispe, M.L. Canonical and noncanonical vascular endothelial growth factor pathways: New developments in biology and signal transduction. Arterioscler. Thromb. Vasc. Biology. 2015, 35, 30–39. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Adamis, A.P. Ten years of anti-vascular endothelial growth factor therapy. Nat. Rev. Drug Discov. 2016, 15, 385–403. [Google Scholar] [CrossRef] [Green Version]
- Makinen, T.; Olofsson, B.; Karpanen, T.; Hellman, U.; Soker, S.; Klagsbrun, M.; Eriksson, U.; Alitalo, K. Differential binding of vascular endothelial growth factor B splice and proteolytic isoforms to neuropilin-1. J. Biol. Chem. 1999, 274, 21217–21222. [Google Scholar] [CrossRef] [Green Version]
- Migdal, M.; Huppertz, B.; Tessler, S.; Comforti, A.; Shibuya, M.; Reich, R.; Baumann, H.; Neufeld, G. Neuropilin-1 is a placenta growth factor-2 receptor. J. Biol. Chem. 1998, 273, 22272–22278. [Google Scholar] [CrossRef] [Green Version]
- Dewerchin, M.; Carmeliet, P. PlGF: A multitasking cytokine with disease-restricted activity. Cold Spring Harb Perspect. Med. 2012, 2, a011056. [Google Scholar] [CrossRef]
- Wang, J.; Huang, Y.; Zhang, J.; Xing, B.; Xuan, W.; Wang, H.; Huang, H.; Yang, J.; Tang, J. NRP-2 in tumor lymphangiogenesis and lymphatic metastasis. Cancer Lett. 2018, 418, 176–184. [Google Scholar] [CrossRef]
- Niland, S.; Eble, J.A. Neuropilins in the context of tumor vasculature. Int. J. Mol. Sci. 2019, 20, 639. [Google Scholar] [CrossRef] [Green Version]
- Park, S.A.; Jeong, M.S.; Ha, K.T.; Jang, S.B. Structure and function of vascular endothelial growth factor and its receptor system. BMB Rep. 2018, 51, 73–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pajusola, K.; Aprelikova, O.; Korhonen, J.; Kaipainen, A.; Pertovaara, L.; Alitalo, R.; Alitalo, K. FLT4 receptor tyrosine kinase contains seven immunoglobulin-like loops and is expressed in multiple human tissues and cell lines. Cancer Res. 1992, 52, 5738–5743. [Google Scholar] [PubMed]
- Apte, R.S.; Chen, D.S.; Ferrara, N. VEGF in signaling and disease: Beyond discovery and development. Cell 2019, 176, 1248–1264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zachary, I. Neuropilins: Role in signalling, angiogenesis and disease. Chem. Immunol. Allergy 2014, 99, 37–70. [Google Scholar] [PubMed] [Green Version]
- Pellet-Many, C.; Frankel, P.; Jia, H.; Zachary, I. Neuropilins: Structure, function and role in disease. Biochem. J. 2008, 411, 211–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Djordjevic, S.; Driscoll, P.C. Targeting VEGF signalling via the neuropilin co-receptor. Drug Discov. Today. 2013, 18, 447–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulpice, E.; Plouet, J.; Berge, M.; Allanic, D.; Tobelem, G.; Merkulova-Rainon, T. Neuropilin-1 and neuropilin-2 act as coreceptors, potentiating proangiogenic activity. Blood 2008, 111, 2036–2045. [Google Scholar] [CrossRef]
- Pan, Q.; Chathery, Y.; Wu, Y.; Rathore, N.; Tong, R.K.; Peale, F.; Bagri, A.; Tessier-Lavigne, M.; Koch, A.W.; Watts, R.J. Neuropilin-1 binds to VEGF121 and regulates endothelial cell migration and sprouting. J. Biol. Chem. 2007, 282, 24049–24056. [Google Scholar] [CrossRef] [Green Version]
- Sarabipour, S.; Mac Gabhann, F. VEGF-A121a binding to neuropilins—A concept revisited. Cell Adh. Migr. 2018, 12, 204–214. [Google Scholar] [CrossRef]
- Adamis, A.P.; Shima, D.T. The role of vascular endothelial growth factor in ocular health and disease. Retina 2005, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Rapisarda, A.; Melillo, G. Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv. Cancer Res. 2012, 114, 237–267. [Google Scholar]
- Barratt, S.L.; Flower, V.A.; Pauling, J.D.; Millar, A.B. VEGF (vascular endothelial growth factor) and fibrotic lung disease. Int. J. Mol. Sci. 2018, 19, 1269. [Google Scholar] [CrossRef] [Green Version]
- Bry, M.; Kivela, R.; Leppanen, V.M.; Alitalo, K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 2014, 94, 779–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrara, N.; Hillan, K.J.; Gerber, H.P.; Novotny, W. Discovery and development of bevacizumab, an anti-VEGF antibody for treating cancer. Nat. Rev. Drug Discov. 2004, 3, 391–400. [Google Scholar] [CrossRef]
- Presta, L.G.; Chen, H.; O’Connor, S.J.; Chisholm, V.; Meng, Y.G.; Krummen, L.; Winkler, M.; Ferrara, N. Humanization of an anti-vascular endothelial growth factor monoclonal antibody for the therapy of solid tumors and other disorders. Cancer Res. 1997, 57, 4593–4599. [Google Scholar]
- Riccardi, C.; Napolitano, E.; Platella, C.; Musumeci, D.; Melone, M.A.B.; Montesarchio, D. Anti-VEGF DNA-based aptamers in cancer therapeutics and diagnostics. Med. Res. Rev. 2020, 41, 464–506. [Google Scholar] [CrossRef]
- Folkman, J. Tumor angiogenesis: Therapeutic implications. N. Engl. J. Med. 1971, 285, 1182–1186. [Google Scholar] [PubMed]
- Muller, Y.A.; Li, B.; Christinger, H.W.; Wells, J.A.; Cunningham, B.C.; de Vos, A.M. Vascular endothelial growth factor: Crystal structure and functional mapping of the kinase domain receptor binding site. Proc. Natl. Acad. Sci. USA 1997, 94, 7192–7197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muller, Y.A.; Christinger, H.W.; Keyt, B.A.; de Vos, A.M. The crystal structure of vascular endothelial growth factor (VEGF) refined to 1.93 A resolution: Multiple copy flexibility and receptor binding. Structure 1997, 5, 1325–1338. [Google Scholar] [CrossRef] [Green Version]
- Starovasnik, M.A.; Christinger, H.W.; Wiesmann, C.; Champe, M.A.; de Vos, A.M.; Skelton, N.J. Solution structure of the VEGF-binding domain of Flt-1: Comparison of its free and bound states. J. Mol. Biol. 1999, 293, 531–544. [Google Scholar] [CrossRef] [PubMed]
- Fairbrother, W.J.; Champe, M.A.; Christinger, H.W.; Keyt, B.A.; Starovasnik, M.A. Solution structure of the heparin-binding domain of vascular endothelial growth factor. Structure 1998, 6, 637–648. [Google Scholar] [CrossRef]
- Stauffer, M.E.; Skelton, N.J.; Fairbrothe, W.J. Refinement of the solution structure of the heparin-binding domain of vascular endothelial growth factor using residual dipolar couplings. J. Biomol. NMR 2002, 23, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Millauer, B.; Wizigmann-Voos, S.; Schnurch, H.; Martinez, R.; Moller, N.P.; Risau, W.; Ullrich, A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell 1993, 72, 835–846. [Google Scholar] [CrossRef]
- Terman, B.I.; Dougher-Vermazen, M.; Carrion, M.E.; Dimitrov, D.; Armellino, D.C.; Gospodarowicz, D.; Bohlen, P. Identification of the KDR tyrosine kinase as a receptor for vascular endothelial cell growth factor. Biochem. Biophys. Res. Commun. 1992, 187, 1579–1586. [Google Scholar] [CrossRef]
- de Vries, C.; Escobedo, J.A.; Ueno, H.; Houck, K.; Ferrara, N.; Williams, L.T. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science 1992, 255, 989–991. [Google Scholar] [CrossRef]
- Shibuya, M. Differential roles of vascular endothelial growth factor receptor-1 and receptor-2 in angiogenesis. J. Biochem. Mol. Biol. 2006, 39, 469–478. [Google Scholar] [CrossRef]
- Davis-Smyth, T.; Chen, H.; Park, J.; Presta, L.G.; Ferrara, N. The second immunoglobulin-like domain of the VEGF tyrosine kinase receptor Flt-1 determines ligand binding and may initiate a signal transduction cascade. EMBO J. 1996, 15, 4919–4927. [Google Scholar] [CrossRef]
- Wiesmann, C.; Fuh, G.; Christinger, H.W.; Eigenbrot, C.; Wells, J.A.; de Vos, A.M. Crystal structure at 1.7 A resolution of VEGF in complex with domain 2 of the Flt-1 receptor. Cell 1997, 91, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Markovic-Mueller, S.; Stuttfeld, E.; Asthana, M.; Weinert, T.; Bliven, S.; Goldie, K.N.; Kisko, K.; Capitani, G.; Ballmer-Hofer, K. Structure of the full-length VEGFR-1 extracellular domain in complex with VEGF-A. Structure 2017, 25, 341–352. [Google Scholar] [CrossRef] [Green Version]
- Fuh, G.; Li, B.; Crowley, C.; Cunningham, B.; Wells, J.A. Requirements for binding and signaling of the kinase domain receptor for vascular endothelial growth factor. J. Biol. Chem. 1998, 273, 11197–11204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brozzo, M.S.; Bjelic, S.; Kisko, K.; Schleier, T.; Leppanen, V.M.; Alitalo, K.; Winkler, F.K.; Ballmer-Hofer, K. Thermodynamic and structural description of allosterically regulated VEGFR-2 dimerization. Blood 2012, 119, 1781–1788. [Google Scholar] [CrossRef]
- Aiello, L.P.; Pierce, E.A.; Foley, E.D.; Takagi, H.; Chen, H.; Riddle, L.; Ferrara, N.; King, G.L.; Smith, L.E. Suppression of retinal neovascularization in vivo by inhibition of vascular endothelial growth factor (VEGF) using soluble VEGF-receptor chimeric proteins. Proc. Natl. Acad. Sci. USA 1995, 92, 10457–10461. [Google Scholar] [CrossRef] [Green Version]
- Pechan, P.; Rubin, H.; Lukason, M.; Ardinger, J.; DuFresne, E.; Hauswirth, W.W.; Wadsworth, S.C.; Scaria, A. Novel anti-VEGF chimeric molecules delivered by AAV vectors for inhibition of retinal neovascularization. Gene Ther. 2009, 16, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Stefano, J.E.; Bird, J.; Kyazike, J.; Cheng, A.W.; Boudanova, E.; Dwyer, M.; Hou, L.; Qiu, H.; Matthews, G.; O’Callaghan, M.; et al. High-affinity VEGF antagonists by oligomerization of a minimal sequence VEGF-binding domain. Bioconjug Chem. 2012, 23, 2354–2364. [Google Scholar] [CrossRef] [PubMed]
- Holash, J.; Davis, S.; Papadopoulos, N.; Croll, S.D.; Ho, L.; Russell, M.; Boland, P.; Leidich, R.; Hylton, D.; Burova, E.; et al. VEGF-Trap: A VEGF blocker with potent antitumor effects. Proc. Natl. Acad. Sci. USA 2002, 99, 11393–11398. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.W.; Xu, P.; Li, X.; Vander Kooi, C.W. Structural basis for selective vascular endothelial growth factor-A (VEGF-A) binding to neuropilin-1. J. Biol. Chem. 2012, 287, 11082–11089. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.J.; Li, B.; Winer, J.; Armanini, M.; Gillett, N.; Phillips, H.S.; Ferrara, N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature 1993, 362, 841–844. [Google Scholar] [CrossRef]
- Muller, Y.A.; Chen, Y.; Christinger, H.W.; Li, B.; Cunningham, B.C.; Lowman, H.B.; de Vos, A.M. VEGF and the Fab fragment of a humanized neutralizing antibody: Crystal structure of the complex at 2.4 A resolution and mutational analysis of the interface. Structure 1998, 6, 1153–1167. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Wang, X.; Fuh, G.; Yu, L.; Wakshull, E.; Khosraviani, M.; Day, E.S.; Demeule, B.; Liu, J.; Shire, S.J.; et al. Comparison of binding characteristics and in vitro activities of three inhibitors of vascular endothelial growth factor A. Mol. Pharm. 2014, 11, 3421–3430. [Google Scholar] [CrossRef]
- Christinger, H.W.; Fuh, G.; de Vos, A.M.; Wiesmann, C. The crystal structure of placental growth factor in complex with domain 2 of vascular endothelial growth factor receptor-1. J. Biol. Chem. 2004, 279, 10382–10388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppanen, V.M.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Kalkkinen, N.; Strandin, T.; Lankinen, H.; Goldman, A.; Ballmer-Hofer, K.; Alitalo, K. Structural determinants of growth factor binding and specificity by VEGF receptor 2. Proc. Natl. Acad. Sci. USA 2010, 107, 2425–2430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Wiesmann, C.; Fuh, G.; Li, B.; Christinger, H.W.; McKay, P.; de Vos, A.M.; Lowman, H.B. Selection and analysis of an optimized anti-VEGF antibody: Crystal structure of an affinity-matured Fab in complex with antigen. J. Mol. Biol. 1999, 293, 865–881. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckmann, R.; Jensen, K.; Fenn, S.; Speck, J.; Krause, K.; Meier, A.; Roth, M.; Fauser, S.; Kimbung, R.; Logan, D.T.; et al. DutaFabs are engineered therapeutic Fab fragments that can bind two targets simultaneously. Nat. Commun. 2021, 12, 708. [Google Scholar] [CrossRef]
- Walker, A.; Chung, C.W.; Neu, M.; Burman, M.; Batuwangala, T.; Jones, G.; Tang, C.M.; Steward, M.; Mullin, M.; Tournier, N.; et al. Novel interaction mechanism of a domain antibody-based inhibitor of human vascular endothelial growth factor with greater potency than ranibizumab and bevacizumab and improved capacity over aflibercept. J. Biol. Chem. 2016, 291, 5500–5511. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.V.; Liang, W.C.; Dennis, M.S.; Eigenbrot, C.; Sidhu, S.S.; Fuh, G. High-affinity human antibodies from phage-displayed synthetic Fab libraries with a single framework scaffold. J. Mol. Biol. 2004, 340, 1073–1093. [Google Scholar] [CrossRef]
- Fuh, G.; Wu, P.; Liang, W.C.; Ultsch, M.; Lee, C.V.; Moffat, B.; Wiesmann, C. Structure-function studies of two synthetic anti-vascular endothelial growth factor Fabs and comparison with the Avastin Fab. J. Biol. Chem. 2006, 281, 6625–6631. [Google Scholar] [CrossRef] [Green Version]
- Fellouse, F.A.; Wiesmann, C.; Sidhu, S.S. Synthetic antibodies from a four-amino-acid code: A dominant role for tyrosine in antigen recognition. Proc. Natl. Acad. Sci. USA 2004, 101, 12467–12472. [Google Scholar] [CrossRef] [Green Version]
- Fellouse, F.A.; Esaki, K.; Birtalan, S.; Raptis, D.; Cancasci, V.J.; Koide, A.; Jhurani, P.; Vasser, M.; Wiesmann, C.; Kossiakoff, A.A.; et al. High-throughput generation of synthetic antibodies from highly functional minimalist phage-displayed libraries. J. Mol. Biol. 2007, 373, 924–940. [Google Scholar] [CrossRef]
- Wiesmann, C.; Christinger, H.W.; Cochran, A.G.; Cunningham, B.C.; Fairbrother, W.J.; Keenan, C.J.; Meng, G.; de Vos, A.M. Crystal structure of the complex between VEGF and a receptor-blocking peptide. Biochemistry 1998, 37, 17765–17772. [Google Scholar] [CrossRef]
- Pan, B.; Li, B.; Russell, S.J.; Tom, J.Y.; Cochran, A.G.; Fairbrother, W.J. Solution structure of a phage-derived peptide antagonist in complex with vascular endothelial growth factor. J. Mol. Biol. 2002, 316, 769–787. [Google Scholar] [CrossRef]
- Mandal, K.; Uppalapati, M.; Ault-Riche, D.; Kenney, J.; Lowitz, J.; Sidhu, S.S.; Kent, S.B. Chemical synthesis and X-ray structure of a heterochiral {D-protein antagonist plus vascular endothelial growth factor} protein complex by racemic crystallography. Proc. Natl. Acad. Sci. USA 2012, 109, 14779–14784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uppalapati, M.; Lee, D.J.; Mandal, K.; Li, H.; Miranda, L.P.; Lowitz, J.; Kenney, J.; Adams, J.J.; Ault-Riche, D.; Kent, S.B.; et al. A potent D-protein antagonist of VEGF-A is nonimmunogenic, metabolically stable, and longer-circulating in vivo. ACS Chem. Biol. 2016, 11, 1058–1065. [Google Scholar] [CrossRef]
- Fedorova, A.; Zobel, K.; Gill, H.S.; Ogasawara, A.; Flores, J.E.; Tinianow, J.N.; Vanderbilt, A.N.; Wu, P.; Meng, Y.G.; Williams, S.P.; et al. The development of peptide-based tools for the analysis of angiogenesis. Chem. Biol. 2011, 18, 839–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Checco, J.W.; Kreitler, D.F.; Thomas, N.C.; Belair, D.G.; Rettko, N.J.; Murphy, W.L.; Forest, K.T.; Gellman, S.H. Targeting diverse protein-protein interaction interfaces with alpha/beta-peptides derived from the Z-domain scaffold. Proc. Natl. Acad. Sci. USA 2015, 112, 4552–4557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iyer, S.; Darley, P.I.; Acharya, K.R. Structural insights into the binding of vascular endothelial growth factor-B by VEGFR-1(D2): Recognition and specificity. J. Biol. Chem. 2010, 285, 23779–23789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leonard, P.; Scotney, P.D.; Jabeen, T.; Iyer, S.; Fabri, L.J.; Nash, A.D.; Acharya, K.R. Crystal structure of vascular endothelial growth factor-B in complex with a neutralising antibody Fab fragment. J. Mol. Biol. 2008, 384, 1203–1217. [Google Scholar] [CrossRef] [PubMed]
- Leppanen, V.M.; Tvorogov, D.; Kisko, K.; Prota, A.E.; Jeltsch, M.; Anisimov, A.; Markovic-Mueller, S.; Stuttfeld, E.; Goldie, K.N.; Ballmer-Hofer, K.; et al. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc. Natl. Acad. Sci. USA 2013, 110, 12960–12965. [Google Scholar] [CrossRef] [Green Version]
- Parker, M.W.; Linkugel, A.D.; Goel, H.L.; Wu, T.; Mercurio, A.M.; Vander Kooi, C.W. Structural basis for VEGF-C binding to neuropilin-2 and sequestration by a soluble splice form. Structure 2015, 23, 677–687. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.C.; Wu, X.; Peale, F.V.; Lee, C.V.; Meng, Y.G.; Gutierrez, J.; Fu, L.; Malik, A.K.; Gerber, H.P.; Ferrara, N.; et al. Cross-species vascular endothelial growth factor (VEGF)-blocking antibodies completely inhibit the growth of human tumor xenografts and measure the contribution of stromal VEGF. J. Biol. Chem. 2006, 281, 951–961. [Google Scholar] [CrossRef] [Green Version]
- Khalili, H.; Brocchini, S.; Khaw, P.T.; Filippov, S.K. Comparative thermodynamic analysis in solution of a next generation antibody mimetic to VEGF. RSC Adv. 2018, 8, 35787–35793. [Google Scholar] [CrossRef]
- Adamson, P.; Wilde, T.; Dobrzynski, E.; Sychterz, C.; Polsky, R.; Kurali, E.; Haworth, R.; Tang, C.M.; Korczynska, J.; Cook, F.; et al. Single ocular injection of a sustained-release anti-VEGF delivers 6months pharmacokinetics and efficacy in a primate laser CNV model. J. Control. Release Off. J. Control. Release Soc. 2016, 244, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Sidhu, S.S.; Li, B.; Chen, Y.; Fellouse, F.A.; Eigenbrot, C.; Fuh, G. Phage-displayed antibody libraries of synthetic heavy chain complementarity determining regions. J. Mol. Biol. 2004, 338, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Dugel, P.U.; Koh, A.; Ogura, Y.; Jaffe, G.J.; Schmidt-Erfurth, U.; Brown, D.M.; Gomes, A.V.; Warburton, J.; Weichselberger, A.; Holz, F.G.; et al. HAWK and HARRIER: Phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology 2020, 127, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.D.; Das, A.; Do, D.V.; Dugel, P.U.; Gomes, A.; Holz, F.G.; Koh, A.; Pan, C.K.; Sepah, Y.J.; Patel, N.; et al. Brolucizumab: Evolution through preclinical and clinical studies and the implications for the management of neovascular age-related macular degeneration. Ophthalmology 2020, 127, 963–976. [Google Scholar] [CrossRef]
- Dugel, P.U.; Singh, R.P.; Koh, A.; Ogura, Y.; Weissgerber, G.; Gedif, K.; Jaffe, G.J.; Tadayoni, R.; Schmidt-Erfurth, U.; Holz, F.G. HAWK and HARRIER: Ninety-six-week outcomes from the phase 3 trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2021, 128, 89–99. [Google Scholar] [CrossRef]
- Arezumand, R.; Alibakhshi, A.; Ranjbari, J.; Ramazani, A.; Muyldermans, S. Nanobodies As Novel Agents for Targeting Angiogenesis in Solid Cancers. Front. Immunol. 2017, 8, 1746. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimizadeh, W.; Mousavi Gargari, S.L.; Javidan, Z.; Rajabibazl, M. Production of novel VHH nanobody inhibiting angiogenesis by targeting binding site of VEGF. Appl. Biochem. Biotechnol. 2015, 176, 1985–1995. [Google Scholar] [CrossRef]
- Ahadi, M.; Ghasemian, H.; Behdani, M.; Kazemi-Lomedasht, F. Oligoclonal selection of nanobodies targeting vascular endothelial growth factor. J. Immunotoxicol. 2019, 16, 34–42. [Google Scholar] [CrossRef] [Green Version]
- Karami, E.; Sabatier, J.M.; Behdani, M.; Irani, S.; Kazemi-Lomedasht, F. A nanobody-derived mimotope against VEGF inhibits cancer angiogenesis. J. Enzym. Inhib. Med. Chem. 2020, 35, 1233–1239. [Google Scholar] [CrossRef]
- Ruckman, J.; Green, L.S.; Beeson, J.; Waugh, S.; Gillette, W.L.; Henninger, D.D.; Claesson-Welsh, L.; Janjic, N. 2’-Fluoropyrimidine RNA-based aptamers to the 165-amino acid form of vascular endothelial growth factor (VEGF165). Inhibition of receptor binding and VEGF-induced vascular permeability through interactions requiring the exon 7-encoded domain. J. Biol. Chem. 1998, 273, 20556–20567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Empting, M. Chapter 1: An introduction to cyclic peptides. In Cyclic Peptides: From Bioorganic Synthesis to Applications; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–14. [Google Scholar]
- Checco, J.W.; Gellman, S.H. Iterative nonproteinogenic residue incorporation yields alpha/beta-peptides with a helix-loop-helix tertiary structure and high affinity for VEGF. Chembiochem A Eur. J. Chem. Biol. 2017, 18, 291–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stahl, A.; Stumpp, M.T.; Schlegel, A.; Ekawardhani, S.; Lehrling, C.; Martin, G.; Gulotti-Georgieva, M.; Villemagne, D.; Forrer, P.; Agostini, H.T.; et al. Highly potent VEGF-A-antagonistic DARPins as anti-angiogenic agents for topical and intravitreal applications. Angiogenesis 2013, 16, 101–111. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, G.A.; Mason, M.; Christie, L.A.; Hansen, C.; Hernandez, L.M.; Burke, J.; Luhrs, K.A.; Hohman, T.C. Functional characterization of Abicipar-Pegol, an anti-VEGF DARPin therapeutic that potently inhibits angiogenesis and vascular permeability. Investig. Ophthalmol. Vis. Sci. 2018, 59, 5836–5846. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairbrother, W.J.; Christinger, H.W.; Cochran, A.G.; Fuh, G.; Keenan, C.J.; Quan, C.; Shriver, S.K.; Tom, J.Y.; Wells, J.A.; Cunningham, B.C. Novel peptides selected to bind vascular endothelial growth factor target the receptor-binding site. Biochemistry 1998, 37, 17754–17764. [Google Scholar] [CrossRef]
- Pan, B.; Fairbrother, W.J. NMR structural analysis of vascular endothelial growth factor in complex with a phage-derived peptide antagonist. Spectroscopy 2003, 17, 169–181. [Google Scholar] [CrossRef] [Green Version]
- Pan, B.; Fairbrother, W.J. 1H, 13C, and 15N resonance assignment of the vascular endothelial growth factor receptor-binding domain in complex with a receptor-blocking peptide. J. Biomol. NMR 2002, 22, 189–190. [Google Scholar] [CrossRef]
- Guryanov, I.; Korzhikov-Vlakh, V.; Bhattacharya, M.; Biondi, B.; Masiero, G.; Formaggio, F.; Tennikova, T.; Urtti, A. Conformationally constrained peptides with high affinity to the vascular endothelial growth factor. J. Med. Chem. 2021, 64, 10900–10907. [Google Scholar] [CrossRef]
- Dyachenko, A.; Goldflam, M.; Vilaseca, M.; Giralt, E. Molecular recognition at protein surface in solution and gas phase: Five VEGF peptidic ligands show inverse affinity when studied by NMR and CID-MS. Biopolymers 2010, 94, 689–700. [Google Scholar] [CrossRef]
- Marquez, B.V.; Beck, H.E.; Aweda, T.A.; Phinney, B.; Holsclaw, C.; Jewell, W.; Tran, D.; Day, J.J.; Peiris, M.N.; Nwosu, C.; et al. Enhancing peptide ligand binding to vascular endothelial growth factor by covalent bond formation. Bioconjug. Chem. 2012, 23, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Coppock, M.B.; Warner, C.R.; Dorsey, B.; Orlicki, J.A.; Sarkes, D.A.; Lai, B.T.; Pitram, S.M.; Rohde, R.D.; Malette, J.; Wilson, J.A.; et al. Protein catalyzed capture agents with tailored performance for in vitro and in vivo applications. Biopolymers 2017, 108, e22934. [Google Scholar] [CrossRef]
- Marquez, B.V.; Ikotun, O.F.; Parry, J.J.; Rogers, B.E.; Meares, C.F.; Lapi, S.E. Development of a radiolabeled irreversible peptide ligand for PET imaging of vascular endothelial growth factor. J. Nucl. Med. 2014, 55, 1029–1034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haase, H.S.; Peterson-Kaufman, K.J.; Lan Levengood, S.K.; Checco, J.W.; Murphy, W.L.; Gellman, S.H. Extending foldamer design beyond alpha-helix mimicry: Alpha/beta-peptide inhibitors of vascular endothelial growth factor signaling. J. Am. Chem. Soc. 2012, 134, 7652–7655. [Google Scholar] [CrossRef] [Green Version]
- Reille-Seroussi, M.; Gaucher, J.F.; Desole, C.; Gagey-Eilstein, N.; Brachet, F.; Broutin, I.; Vidal, M.; Broussy, S. Vascular endothelial growth factor peptide ligands explored by competition assay and isothermal titration calorimetry. Biochemistry 2015, 54, 5147–5156. [Google Scholar] [CrossRef] [PubMed]
- Kenrick, S.A.; Daugherty, P.S. Bacterial display enables efficient and quantitative peptide affinity maturation. Protein. Eng. Des. Sel. 2010, 23, 9–17. [Google Scholar] [CrossRef]
- Jurrus, E.; Engel, D.; Star, K.; Monson, K.; Brandi, J.; Felberg, L.E.; Brookes, D.H.; Wilson, L.; Chen, J.; Liles, K.; et al. Improvements to the APBS biomolecular solvation software suite. Protein Sci. Publ. Protein Soc. 2018, 27, 112–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayo-Puxan, N.; Rodriguez-Mias, R.; Goldflam, M.; Kotev, M.; Ciudad, S.; Hipolito, C.J.; Varese, M.; Suga, H.; Campos-Olivas, R.; Barril, X.; et al. Combined use of oligopeptides, fragment libraries, and natural compounds: A comprehensive approach to sample the druggability of vascular endothelial growth factor. ChemMedChem. 2016, 11, 928–939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krissinel, E.; Henrick, K. Inference of macromolecular assemblies from crystalline state. J. Mol. Biol. 2007, 372, 774–797. [Google Scholar] [CrossRef] [PubMed]
- Iyer, S.; Leonidas, D.D.; Swaminathan, G.J.; Maglione, D.; Battisti, M.; Tucci, M.; Persico, M.G.; Acharya, K.R. The crystal structure of human placenta growth factor-1 (PlGF-1), an angiogenic protein, at 2.0 A resolution. J. Biol. Chem. 2001, 276, 12153–12161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anisimov, A.; Leppanen, V.M.; Tvorogov, D.; Zarkada, G.; Jeltsch, M.; Holopainen, T.; Kaijalainen, S.; Alitalo, K. The basis for the distinct biological activities of vascular endothelial growth factor receptor-1 ligands. Sci. Signal. 2013, 6, ra52. [Google Scholar] [CrossRef] [Green Version]
- Fischer, C.; Jonckx, B.; Mazzone, M.; Zacchigna, S.; Loges, S.; Pattarini, L.; Chorianopoulos, E.; Liesenborghs, L.; Koch, M.; De Mol, M.; et al. Anti-PlGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 2007, 131, 463–475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van de Veire, S.; Stalmans, I.; Heindryckx, F.; Oura, H.; Tijeras-Raballand, A.; Schmidt, T.; Loges, S.; Albrecht, I.; Jonckx, B.; Vinckier, S.; et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell 2010, 141, 178–190. [Google Scholar] [CrossRef] [Green Version]
- Bais, C.; Wu, X.; Yao, J.; Yang, S.; Crawford, Y.; McCutcheon, K.; Tan, C.; Kolumam, G.; Vernes, J.M.; Eastham-Anderson, J.; et al. PlGF blockade does not inhibit angiogenesis during primary tumor growth. Cell 2010, 141, 166–177. [Google Scholar] [CrossRef] [Green Version]
- Yao, J.; Wu, X.; Zhuang, G.; Kasman, I.M.; Vogt, T.; Phan, V.; Shibuya, M.; Ferrara, N.; Bais, C. Expression of a functional VEGFR-1 in tumor cells is a major determinant of anti-PlGF antibodies efficacy. Proc. Natl. Acad. Sci. USA 2011, 108, 11590–11595. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Snuderl, M.; Batista, A.; Kirkpatrick, N.D.; Ruiz de Almodovar, C.; Riedemann, L.; Walsh, E.C.; Anolik, R.; Huang, Y.; Martin, J.D.; Kamoun, W.; et al. Targeting placental growth factor/neuropilin 1 pathway inhibits growth and spread of medulloblastoma. Cell 2013, 152, 1065–1076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Albonici, L.; Giganti, M.G.; Modesti, A.; Manzari, V.; Bei, R. Multifaceted role of the placental growth Factor (PlGF) in the antitumor immune response and cancer progression. Int. J. Mol. Sci. 2019, 20, 2970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassen, U.; Nielsen, D.L.; Sorensen, M.; Winstedt, L.; Niskanen, T.; Stenberg, Y.; Pakola, S.; Stassen, J.M.; Glazer, S. A phase I, dose-escalation study of TB-403, a monoclonal antibody directed against PlGF, in patients with advanced solid tumours. Br. J. Cancer 2012, 106, 678–684. [Google Scholar] [CrossRef] [Green Version]
- Arezumand, R.; Mahdian, R.; Zeinali, S.; Hassanzadeh-Ghassabeh, G.; Mansouri, K.; Khanahmad, H.; Namvar-Asl, N.; Rahimi, H.; Behdani, M.; Cohan, R.A.; et al. Identification and characterization of a novel nanobody against human placental growth factor to modulate angiogenesis. Mol. Immunol. 2016, 78, 183–192. [Google Scholar] [CrossRef]
- Nikooharf, A.; Arezumand, R.; Mansouri, K.; Khoshi, A.H.; Namdar Ahmadabad, H. Development of a recombinant monospecific anti-PLGF bivalent nanobody and evaluation of it in angiogenesis modulation. Mol. Biotechnol. 2020, 62, 580–588. [Google Scholar] [CrossRef]
- Scotney, P.D.; MacKenzie, A.; Maccarone, P.; Fabri, L.J.; Scrofani, S.D.; Gooley, P.R.; Nash, A.D. Human vascular endothelial growth factor B: Characterization of recombinant isoforms and generation of neutralizing monoclonal antibodies. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1024–1029. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.; Tang, Z.; Hou, X.; Lennartsson, J.; Li, Y.; Koch, A.W.; Scotney, P.; Lee, C.; Arjunan, P.; Dong, L.; et al. VEGF-B is dispensable for blood vessel growth but critical for their survival, and VEGF-B targeting inhibits pathological angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 6152–6157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irani, Y.D.; Scotney, P.D.; Klebe, S.; Mortimer, L.A.; Nash, A.D.; Williams, K.A. An anti-VEGF-B antibody fragment induces regression of pre-existing blood vessels in the rat cornea. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3404–3413. [Google Scholar] [CrossRef] [Green Version]
- Irani, Y.; Scotney, P.; Nash, A.; Williams, K.A. Species cross-reactivity of antibodies used to treat ophthalmic conditions. Investig. Ophthalmol. Vis. Sci. 2016, 57, 586–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bower, K.E.; Lam, S.N.; Oates, B.D.; Del Rosario, J.R.; Corner, E.; Osothprarop, T.F.; Kinhikar, A.G.; Hoye, J.A.; Preston, R.R.; Murphy, R.E.; et al. Evolution of potent and stable placental-growth-factor-1-targeting CovX-bodies from phage display peptide discovery. J. Med. Chem. 2011, 54, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Davydova, N.; Harris, N.C.; Roufail, S.; Paquet-Fifield, S.; Ishaq, M.; Streltsov, V.A.; Williams, S.P.; Karnezis, T.; Stacker, S.A.; Achen, M.G. Differential receptor binding and regulatory mechanisms for the lymphangiogenic growth factors vascular endothelial growth factor (VEGF)-C and -D. J. Biol. Chem. 2016, 291, 27265–27278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leppanen, V.M.; Jeltsch, M.; Anisimov, A.; Tvorogov, D.; Aho, K.; Kalkkinen, N.; Toivanen, P.; Yla-Herttuala, S.; Ballmer-Hofer, K.; Alitalo, K. Structural determinants of vascular endothelial growth factor-D receptor binding and specificity. Blood 2011, 117, 1507–1515. [Google Scholar] [CrossRef] [Green Version]
- Stacker, S.A.; Achen, M.G. Emerging roles for VEGF-D in human disease. Biomolecules 2018, 8, 1. [Google Scholar] [CrossRef] [Green Version]
- Dugel, P.U.; Boyer, D.S.; Antoszyk, A.N.; Steinle, N.C.; Varenhorst, M.P.; Pearlman, J.A.; Gillies, M.C.; Finger, R.P.; Baldwin, M.E.; Leitch, I.M. Phase 1 study of OPT-302 inhibition of vascular endothelial growth factors C and D for neovascular age-related macular degeneration. Ophthalmol. Retin. 2020, 4, 250–263. [Google Scholar] [CrossRef]
- Timoshenko, A.V.; Rastogi, S.; Lala, P.K. Migration-promoting role of VEGF-C and VEGF-C binding receptors in human breast cancer cells. Br. J. Cancer 2007, 97, 1090–1098. [Google Scholar] [CrossRef]
- Kampen, K.R.; Scherpen, F.J.G.; Mahmud, H.; Ter Elst, A.; Mulder, A.B.; Guryev, V.; Verhagen, H.; De Keersmaecker, K.; Smit, L.; Kornblau, S.M.; et al. VEGFC antibody therapy drives differentiation of AML. Cancer Res. 2018, 78, 5940–5948. [Google Scholar] [CrossRef] [Green Version]
- Hajrasouliha, A.R.; Funaki, T.; Sadrai, Z.; Hattori, T.; Chauhan, S.K.; Dana, R. Vascular endothelial growth factor-C promotes alloimmunity by amplifying antigen-presenting cell maturation and lymphangiogenesis. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1244–1250. [Google Scholar] [CrossRef] [Green Version]
- Goyal, S.; Chauhan, S.K.; Dana, R. Blockade of prolymphangiogenic vascular endothelial growth factor C in dry eye disease. Arch. Ophthalmol. 2012, 130, 84–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Achen, M.G.; Roufail, S.; Domagala, T.; Catimel, B.; Nice, E.C.; Geleick, D.M.; Murphy, R.; Scott, A.M.; Caesar, C.; Makinen, T.; et al. Monoclonal antibodies to vascular endothelial growth factor-D block its interactions with both VEGF receptor-2 and VEGF receptor-3. Eur. J. Biochem. 2000, 267, 2505–2515. [Google Scholar] [CrossRef] [PubMed]
- Davydova, N.; Roufail, S.; Streltsov, V.A.; Stacker, S.A.; Achen, M.G. The VD1 neutralizing antibody to vascular endothelial growth factor-D: Binding epitope and relationship to receptor binding. J. Mol. Biol. 2011, 407, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Wise, L.M.; Inder, M.K.; Real, N.C.; Stuart, G.S.; Fleming, S.B.; Mercer, A.A. The vascular endothelial growth factor (VEGF)-E encoded by orf virus regulates keratinocyte proliferation and migration and promotes epidermal regeneration. Cell. Microbiol. 2012, 14, 1376–1390. [Google Scholar] [CrossRef] [PubMed]
- Fleming, S.B.; Wise, L.M.; Mercer, A.A. Molecular genetic analysis of orf virus: A poxvirus that has adapted to skin. Viruses 2015, 7, 1505–1539. [Google Scholar] [CrossRef]
- Meyer, M.; Clauss, M.; Lepple-Wienhues, A.; Waltenberger, J.; Augustin, H.G.; Ziche, M.; Lanz, C.; Buttner, M.; Rziha, H.J.; Dehio, C. A novel vascular endothelial growth factor encoded by Orf virus, VEGF-E, mediates angiogenesis via signalling through VEGFR-2 (KDR) but not VEGFR-1 (Flt-1) receptor tyrosine kinases. EMBO J. 1999, 18, 363–374. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, S.; Oku, A.; Sawano, A.; Yamaguchi, S.; Yazaki, Y.; Shibuya, M. A novel type of vascular endothelial growth factor, VEGF-E (NZ-7 VEGF), preferentially utilizes KDR/Flk-1 receptor and carries a potent mitotic activity without heparin-binding domain. J. Biol. Chem. 1998, 273, 31273–31282. [Google Scholar] [CrossRef] [Green Version]
- Wise, L.M.; Veikkola, T.; Mercer, A.A.; Savory, L.J.; Fleming, S.B.; Caesar, C.; Vitali, A.; Makinen, T.; Alitalo, K.; Stacker, S.A. Vascular endothelial growth factor (VEGF)-like protein from orf virus NZ2 binds to VEGFR2 and neuropilin-1. Proc. Natl. Acad. Sci. USA 1999, 96, 3071–3076. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, Y.; Matsunaga, Y.; Tokunaga, Y.; Obayashi, S.; Saito, M.; Morita, T. Snake venom Vascular Endothelial Growth Factors (VEGF-Fs) exclusively vary their structures and functions among species. J. Biol. Chem. 2009, 284, 9885–9891. [Google Scholar] [CrossRef] [Green Version]
- Toivanen, P.I.; Nieminen, T.; Laakkonen, J.P.; Heikura, T.; Kaikkonen, M.U.; Yla-Herttuala, S. Snake venom VEGF Vammin induces a highly efficient angiogenic response in skeletal muscle via VEGFR-2/NRP specific signaling. Sci. Rep. 2017, 7, 5525. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamazaki, Y.; Tokunaga, Y.; Takani, K.; Morita, T. C-terminal heparin-binding peptide of snake venom VEGF specifically blocks VEGF-stimulated endothelial cell proliferation. Pathophysiol. Haemost. Thromb. 2005, 34, 197–199. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, Y.; Tokunaga, Y.; Takani, K.; Morita, T. Identification of the heparin-binding region of snake venom vascular endothelial growth factor (VEGF-F) and its blocking of VEGF-A165. Biochemistry 2005, 44, 8858–8864. [Google Scholar] [CrossRef] [PubMed]









| VEGF Member | Ligand | Bound Epitope(s) of VEGF a | Affinity/Method | Reference/PDB Code |
|---|---|---|---|---|
| VEGF-A | VEGFR-1D2 | Monomer one:
| IC50 = 1.4 nM/ELISA type assay with biotinylated VEGF8-109 IC50 = 3.0 nM (VEGF8-109) SPR competition assay [81] | 1FLT [69] or 1QTY [61] |
| VEGFR-2D2-3 | Idem above (helix α1, loops 2 and 3) + loop 1, which interacts solely with D3 | Kd = 170 nM (VEGF165)/ITC: unfavorable binding enthalpy Kd = 18 ± 5.2 nM (VEGF165)/ITC [82] Kd = 36.7 ± 5.9 nM (VEGF165)/SPR [82] | 3V2A [72] | |
| VEGFR-1D1-6 | Residues interacting with D2 Monomer one:
Monomer one:
| Kd = 47 nM (VEGFR-1D1-3)/ITC Kd = 1.7 nM (VEGFR-1D1-7)/ITC | 5T89 [70] | |
| Neuropilin-1 |
| Kd = 3.0 ± 0.2 nM (VEGF164)/ELISA type assay with AP-VEGF164 | 4DEQ [77] | |
| Fab-12 (refer to Y0192 in the article) | Monomer one:
| IC50 = 4.7 nM/Fab-phage ELISA; Kd = 3.4 ± 0.9 nM (25 °C)/SPR [8] Kd = 13 ± 2.2 nM [37 °C, VEGF109]/BIAcore SPR; Kd = 21 ± 3.8 nM [37 °C, VEGF165]/SPR; IC50 = 9 nM (37 °C)/ELISA assay with Fab12-IgG and Biotin-VEGF109; Kd = 0.433 nM (25 °C)/radiolabeled VEGF binding assay using VEGF competition with [125I] VEGF for binding to Fab [83] | 1BJ1 [79] | |
| Y0317-Fab | Same as Fab-12 (binding site centers on the 80′s loop of VEGF) | Kd ≤ 0.14 nM [25 °C, VEGF109]/SPR; Kd = 0.11 ± 0.02 nM [37 °C, VEGF109]/SPR; Kd = 0.14 ± 0.05 nM [37 °C, VEGF165]/SPR;IC50 = 1 nM/ELISA assay with Biotin-VEGF109 Kd = 0.02 nM (25 °C)/radiolabeled VEGF binding assay using VEGF competition with [125I] VEGF for binding to Fab [9] | 1CZ8 [83] | |
| DutaFab |
| IC50 = 34 pM/ELISA assay with VEGF165 | 6T9D [84] | |
| Dual dAb | Similar to VEGFR-1D2 | Mammalian cell-derived hVEGF165: Kd = 3.27 pM; E. coli expressed hVEGF165: Kd = 3.14 pM/T200 SPR hVEGF-A165: EC50 = 32 ± 2.7 pM; hVEGF121: EC50 = 127 ± 22.1 pM; mVEGF164: EC50 = 36 ± 4.7 pM/Mesoscale discovery binding assay VEGFR-1 IC50 = 59 ± 11.8 pM;VEGFR-2 IC50 = 22 ± 2.7 pM/receptor binding assay by Mesoscale discovery | VK·dAb: 5FV1 VH dAb: 5FV2 [85] | |
| G6-Fab | Monomer one:
| Anti-mVEGF IC50 = 0.6 nM/Fab-phage ELISA; Kd = 0.91 nM/SPR [86] | 2FJG [87] | |
| B20-4-Fab | Monomer one:
| Kd = 12 nM | 2FJH [87] | |
| YADS1-Fab | The structural epitopes for binding to YADS1-Fab and YADS2-Fab overlap with each other and also with the structural epitope for binding to VEGFR-1D2 | For hVEGF Kd = 1.8 ± 0.3 nM For mVEGF Kd > 1000 nM/SPR | 1TZH [88] | |
| YADS2-Fab | For hVEGF Kd = 10 ± 2 nM For mVEGF Kd = 5.0 ± 0.8 nM/SPR | 1TZI [88] | ||
| D1-Fab | The structural epitope overlaps with the structural epitope for VEGFR-1D2 | Kd = 7.8 nM/BIAcore SPR | 2QR0 [89] | |
| Peptide v108 | Monomer one:
| IC50 = 8.2 μM/ELISA biotinylated VEGF8-109 [90] | 1VPP [90] | |
| Peptide v107 | Monomer one:
| IC50 = 1 μM/ELISA type assay with biotin labeled-v107 | 1KAT [91] | |
| D-RFX001 | Bind to the same region of VEGF-A that interacts with VEGFR-1D2; cover much of the contact surface that VEGF-A uses to interact with VEGFR-1D2; Surface area of the binding interface is 800 Å2 | Kd = 85 ± 12 nM/SPR, in a ProteOn™ XPR36 Protein Interaction Array System | 4GLN or 4GLS [92] | |
| D-RFX037 | Identical to D-RFX001 Surface area of the binding interface is 1350 Å2 | Kd = 6.43 ± 0.07 nM/SPR, in a ProteOn XPR36 Protein Interaction Array System | 5HHD or 5HHC [93] | |
| Z-Domain | Overlaps with the VEGFR-1D2 binding interface | IC50 = 343 nM/phage ELISA Kd = 38 nM/Octet binding assay | 3S1K [94] | |
| Mini-Z dimer | Overlaps with the VEGFR-1D2 binding interface | IC50 = 227 nM/phage ELISA Kd = 40 nM/Octet binding assay | 3S1B [94] | |
| Alpha/beta | Overlaps with the VEGFR-1D2 binding interface | Ki = 0.11 μM/FP assay | 4WPB [95] | |
| VEGF-B | VEGFR-1D2 | Monomer one:
| 2XAC [96] | |
| 2H10-Fab | Monomer one:
| Kd = 113.7 pM (VEGF-B10-108)/SPR IC50 = 3.4 nM (VEGF-B10-108)/cell-based assay | 2VWE [97] | |
| PlGF | VEGFR-1D2 | Monomer one:
| IC50 = 275 nM (PlGF19-116)/SPR competition assay | 1RV6 [81] |
| VEGF-C | VEGFR-2D2-3 | Monomer one:
| Kd = 16 ± 6.7 nM/ITC with a VEGF-C mutant C137A Kd = 18.2 ± 5.3 nM/SPR with a VEGF-C mutant C137A | 2X1X or 2X1W [82] |
| VEGFR-3D1-2 | The overall complex architecture is very similar to that of previously reported VEGFR-1 and VEGFR-2 structures VEGF-C binding is limited to D2, with D1 protruding away from VEGF-C | Kd = 250 nM/ITC with VEGF-C mutant C137A | 4BSK [98] | |
| Neuropilin-2 (with C-terminus of VEGF-C) | R164 and R165 (VEGF165-A numbering) | ELISA type assay with AP-VEGF-C differential scanning fluorimetry (DSF) thermal shift assay (no data about Kd or IC50 of VEGF-C C-terminus binding to Neuropilin-2) | 4QDQ [99] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, X.; Gaucher, J.-F.; Vidal, M.; Broussy, S. A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules 2021, 26, 6759. https://doi.org/10.3390/molecules26226759
Ye X, Gaucher J-F, Vidal M, Broussy S. A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules. 2021; 26(22):6759. https://doi.org/10.3390/molecules26226759
Chicago/Turabian StyleYe, Xiaoqing, Jean-François Gaucher, Michel Vidal, and Sylvain Broussy. 2021. "A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics" Molecules 26, no. 22: 6759. https://doi.org/10.3390/molecules26226759
APA StyleYe, X., Gaucher, J. -F., Vidal, M., & Broussy, S. (2021). A Structural Overview of Vascular Endothelial Growth Factors Pharmacological Ligands: From Macromolecules to Designed Peptidomimetics. Molecules, 26(22), 6759. https://doi.org/10.3390/molecules26226759