Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species
Abstract
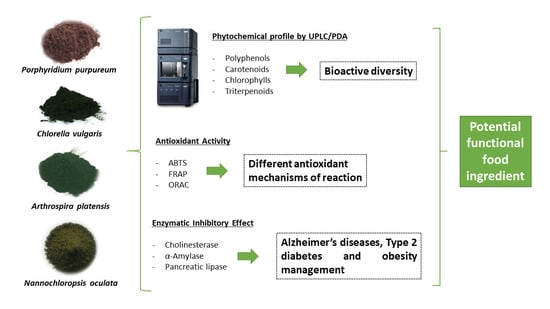
:1. Introduction
2. Results and Discussion
2.1. Determination of Polyphenolic Content
2.2. Determination of Carotenoid and Chlorophyll Content
2.3. Determination of Triterpenoid Content
2.4. Evaluation of the Antioxidant Potential
2.5. Assessment of the Cholinesterase, α-Amylase, and Pancreatic Lipase Enzymatic Inhibition Activities
3. Material and Methods
3.1. Materials
3.2. Determination of Bioactive Content
3.2.1. Determination of Polyphenolic Compounds
3.2.2. Determination of Carotenoids and Chlorophyll Content
3.2.3. Determination of Triterpenoid Compounds
3.3. Preparation of Microalgae Extracts
3.4. Evaluation of Antioxidant Activity
3.5. Determination of Acetylcholinesterase and Butyrylcholinesterase Inhibition
3.6. Determination of the Inhibitory Effect on the Digestives Enzymes α-Amylase and Pancreatic Lipase
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Betoret, E.; Betoret, N.; Vidal, D.; Fito, P. Functional foods development: Trends and technologies. Trends Food Sci. Technol. 2011, 22, 498–508. [Google Scholar] [CrossRef]
- Lordan, S.; Ross, R.P.; Stanton, C. Marine bioactives as functional food ingredients: Potential to reduce the incidence of Chronic Diseases. Mar. Biotechnol. 2011, 9, 1056–1100. [Google Scholar] [CrossRef] [Green Version]
- WHO. Diet, Nutrition and the Prevention of Chronic Diseases; WHO Technical Report Series No. 916; WHO: Geneva, Switzerland, 2003. [Google Scholar]
- Granato, D.; Branco, G.F.; Nazzaro, F.; Faria, A.F. Functional foods and nondairy probiotic food development: Trends, concepts, and products. Compr. Rev. Food Sci. Food Saf. 2010, 9, 292–302. [Google Scholar] [CrossRef]
- Roberfroid, M.B. Concepts and strategy of functional food science: The European perspective. Am. J. Clin. Nutr. 2000, 71, 1660–1664. [Google Scholar] [CrossRef] [Green Version]
- Dewapriya, P.; Kim, S. Marine microorganisms: An emerging avenue in modern nutraceuticals and functional foods. Food Res. Int. 2014, 56, 115–125. [Google Scholar] [CrossRef]
- Plaza, M.; Cifuentes, A.; Ibáñez, E. In the search of new functional food ingredients from algae. Trends Food Sci. Technol. 2008, 19, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Vieira, M.V.; Pastrana, L.M.; Fuciños, P. Microalgae encapsulation systems for food, pharmaceutical and cosmetics applications. Mar. Drugs 2020, 18, 644. [Google Scholar] [CrossRef]
- Rumin, J.; Nicolau, E.; de Oliveira, R.G.; Fuentes-Grünewald, C.; Picot, L. Analysis of scientific research driving microalgae market opportunities in Europe. Mar. Drugs 2020, 18, 264. [Google Scholar] [CrossRef]
- De Morais, M.G.; Vaz, S.; De Morais, E.G.; Alberto, J.; Costa, V. Biologically active metabolites synthesized by microalgae. Biomed Res. Int. 2015, 2015, 835761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyande, A.K.; Chew, K.W.; Rambabu, K.; Tao, Y.; Chu, D.; Show, P. Microalgae: A potential alternative to health supplementation for humans. Food Sci. Hum. Wellness 2019, 8, 16–24. [Google Scholar] [CrossRef]
- de Jesus Raposo, M.F.; De Morais, R.M.S.C.; De Morais, A.M.M.B. Health applications of bioactive compounds from marine microalgae. Life Sci. 2013, 93, 479–486. [Google Scholar] [CrossRef]
- Caporgno, M.P.; Mathys, A. Trends in microalgae incorporation into innovative food products with potential health benefits. Front. Nutr. 2018, 5, 58. [Google Scholar] [CrossRef] [PubMed]
- Borowitzka, M.A. High-value products from microalgae-their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Bernaerts, T.M.M.; Gheysen, L.; Kyomugasho, C.; Jamsazzadeh, Z.; Vandionant, S.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Comparison of microalgal biomasses as functional food ingredients: Focus on the composition of cell wall related polysaccharides. Algal Res. 2018, 32, 150–161. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Matos, Â.P.; Feller, R.; Moecke, E.H.S.; de Oliveira, J.V.; Junior, A.F.; Derner, R.B.; Sant’Anna, E.S. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016, 93, 963–972. [Google Scholar] [CrossRef]
- Tresserra-Rimbau, A.; Lamuela-Raventos, R.M.; Moreno, J.J. Polyphenols, food and pharma. Current knowledge and directions for future. Biochem. Pharmacol. 2018, 156, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Bhuvana, P.; Sangeetha, P.; Anuradha, V.; Ali, M.S. Spectral characterization of bioactive compounds from microalgae: N. oculata and C. vulgaris. Biocatal. Agric. Biotechnol. 2019, 19, 101094. [Google Scholar] [CrossRef]
- da Silva, M.F.; Casazza, A.A.; Ferrari, P.F.; Aliakbarian, B.; Converti, A.; Bezerra, R.P.; Porto, A.L.F.; Perego, P. Recovery of phenolic compounds of food concern from Arthrospira platensis by green extraction techniques. Algal Res. 2017, 25, 391–401. [Google Scholar] [CrossRef]
- Safafar, H.; Van Wagenen, J.; Møller, P.; Jacobsen, C. Carotenoids, phenolic compounds and tocopherols contribute to the antioxidative properties of some microalgae species grown on industrial wastewater. Mar. Drugs 2015, 13, 7339–7356. [Google Scholar] [CrossRef] [Green Version]
- Park, W.S.; Kim, H.; Li, M.; Lim, D.H.; Kim, J.; Kwak, S.; Kang, C.; Ferruzzi, M.G.; Ahn, M. Two classes of pigments, carotenoids and c-phycocyanin, in spirulina powder and their antioxidant activities. Molecules 2018, 23, 2065. [Google Scholar] [CrossRef] [Green Version]
- Papalia, T.; Sidari, R.; Panuccio, M.R. Impact of different storage methods on bioactive compounds in arthrospira platensis biomass. Molecules 2019, 24, 2810. [Google Scholar] [CrossRef] [Green Version]
- Juin, C.; Bonnet, A.; Nicolau, E.; Devillers, R.; Cadoret, J.; Picot, L. UPLC-MSE profiling of phytoplankton metabolites: Application to the identification of pigments and structural analysis of metabolites in Porphyridium purpureum. Mar. Drugs 2015, 13, 2541–2558. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.; Nikolov, Z. Process for selective extraction of pigments and functional proteins from Chlorella vulgaris. Algal Res. 2018, 35, 185–193. [Google Scholar] [CrossRef]
- Dzubak, P.; Hajduch, M.; Vydra, D.; Hustova, A.; Kvasnica, M.; Biedermann, D.; Markova, L.; Sarek, J. Pharmacological activities of natural triterpenoids and their therapeutic implications. Nat. Prod. Rep. 2006, 23, 394–411. [Google Scholar] [CrossRef]
- Sun, H.; Fang, W.; Wang, W.; Hu, C. Structure-activity relationships of oleanane- and ursane-type triterpenoids. Bot. Stud. 2006, 47, 339–368. [Google Scholar]
- Gunes, S.; Tamburaci, S.; Dalay, M.C.; Gurhan, I.D. In vitro evaluation of Spirulina platensis extract incorporated skin cream with its wound healing and antioxidant activities. Pharm. Biol. 2017, 55, 1824–1832. [Google Scholar] [CrossRef] [Green Version]
- Habashy, N.H.; Abu, M.M.; Attia, W.E.; Abdelgaleil, S.A.M. Chemical characterization, antioxidant and anti-inflammatory properties of Greek Thymus vulgaris extracts and their possible synergism with Egyptian Chlorella vulgaris. J. Funct. Foods 2018, 40, 317–328. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Nayak, B.; Liu, R.H.; Tang, J. Effect of processing on phenolic antioxidants of fruits, vegetables, and grains—A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 887–918. [Google Scholar]
- Fotiou, D.; Kaltsatou, A.; Tsiptsios, D.; Nakou, M. Evaluation of the cholinergic hypothesis in Alzheimer’s disease with neuropsychological methods. Aging Clin. Exp. Res. 2015, 27, 727–733. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I.; Koulen, P. Therapeutic potentials of microalgae in the treatment of Alzheimer’s disease. Molecules 2017, 22, 480. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, P.; Wojdyło, A. Content of bioactive compounds in the peach kernels and their antioxidant, anti-hyperglycemic, anti-aging properties. Eur. Food Res. Technol. 2018, 245, 1123–1136. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Justo, T.; Silvestre, L.; Barradas, A.; Duarte, C.V.; Pereira, H.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Microalgae of different phyla display antioxidant, metal chelating and acetylcholinesterase inhibitory activities. Food Chem. 2012, 131, 134–140. [Google Scholar] [CrossRef]
- Tkacz, K.; Wojdyło, A.; Turkiewicz, I.P.; Nowicka, P. Anti-diabetic, anti-cholinesterase, and antioxidant potential, chemical composition and sensory evaluation of novel sea buckthorn-based smoothies. Food Chem. 2021, 338, 128105. [Google Scholar] [CrossRef]
- Işık, M.; Beydemir, Ş. The impact of some phenolic compounds on serum acetylcholinesterase: Kinetic analysis of an enzyme/inhibitor interaction and molecular docking study. J. Biomol. Struct. Dyn. 2020, 39, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Oboh, G.; Agunloye, O.M.; Akinyemi, A.J.; Ademiluyi, A.O.; Adefegha, S.A. Comparative study on the inhibitory effect of caffeic and chlorogenic acids on key enzymes linked to Alzheimer’s disease and some pro-oxidant induced oxidative stress in rats’ brain-in vitro. Neurochem. Res. 2013, 38, 413–419. [Google Scholar] [CrossRef]
- Hira, S.; Saleem, U.; Anwar, F.; Sohail, M.F.; Raza, Z.; Ahmad, B. β-Carotene: A natural compound improves cognitive impairment and oxidative stress in a mouse model of streptozotocin-induced Alzheimer’s disease. Biomolecules 2019, 9, 441. [Google Scholar] [CrossRef] [Green Version]
- Custódio, L.; Soares, F.; Pereira, H.; Rodrigues, M.J.; Barreira, L.; Rauter, A.P.; Alberício, F.; Varela, J. Botryococcus braunii and Nannochloropsis oculata extracts inhibit cholinesterases and protect human dopaminergic SH-SY5Y cells from H2O2-induced cytotoxicity. J. Appl. Phycol. 2015, 27, 839–848. [Google Scholar] [CrossRef]
- Olasehinde, T.A.; Olaniran, A.O.; Okoh, A.I. Cholinesterase inhibitory activity, antioxidant properties, and phytochemical composition of Chlorococcum sp. extracts. J. Food Biochem. 2020, 45, e13395. [Google Scholar] [CrossRef] [PubMed]
- Zelík, P.; Lukešová, A.; Voloshko, L.N.; Štys, D.; Kopecký, J. Screening for acetylcholinesterase inhibitory activity in cyanobacteria of the genus Nostoc. J. Enzyme Inhib. Med. Chem. 2009, 24, 531–536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alzahrani, M.A.J.; Perera, C.O.; Sabaragamuwa, R.; Hemar, Y. Assessment of bioactive potential of aqueous protein extracts from Diatoms Nitzschia laevis, Spirulina platensis, and Chlorella vulgaris. J. Aquat. Food Prod. Technol. 2019, 28, 177–193. [Google Scholar] [CrossRef]
- Chatterjee, S.; Khunti, K.; Davies, M.J. Type 2 diabetes. Lancet 2017, 389, 2239–2251. [Google Scholar] [CrossRef]
- Striegel, L.; Kang, B.; Pilkenton, S.J.; Rychlik, M.; Apostolidis, E. Effect of black tea and black tea pomace polyphenols on α-glucosidase and α-amylase inhibition, relevant to Type 2 Diabetes prevention. Front. Nutr. 2015, 2, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Nowicka, P.; Wojdyło, A.; Samoticha, J. Evaluation of phytochemicals, antioxidant capacity, and antidiabetic activity of novel smoothies from selected Prunus fruits. J. Funct. Foods 2016, 25, 397–407. [Google Scholar] [CrossRef]
- Nowicka, P.; Wojdyło, A.; Laskowski, P. Inhibitory potential against digestive enzymes linked to obesity and type 2 diabetes and content of bioactive compounds in 20 cultivars of the peach fruit grown in poland. Plant Foods Hum. Nutr. 2018, 73, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Sathasivam, R.; Ki, J.S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Mar. Drugs 2018, 16, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roohbakhsh, A.; Karimi, G.; Iranshahi, M. Carotenoids in the treatment of diabetes mellitus and its complications: A mechanistic review. Biomed. Pharmacother. 2017, 91, 31–42. [Google Scholar] [CrossRef]
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, hypoglycaemic and hypolipidaemic effects of bioactive compounds from microalgae and marine. Int. J. Food Sci. Technol. 2015, 50, 1705–1717. [Google Scholar] [CrossRef]
- Ramos-Romero, S.; Torrella, J.R.; Pagès, T.; Viscor, G.; Torres, J.L. Edible microalgae and their bioactive compounds in the prevention and treatment of metabolic alterations. Nutrients 2021, 13, 563. [Google Scholar] [CrossRef]
- Hu, S.; Fan, X.; Qi, P.; Zhang, X. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Munawaroh, H.S.H.; Gumilar, G.G.; Nurjanah, F.; Yuliani, G.; Aisyah, S.; Kurnia, D.; Wulandari, A.P.; Kurniawan, I.; Ningrum, A.; Koyande, A.K.; et al. In-vitro molecular docking analysis of microalgae extracted phycocyanin as an anti-diabetic candidate. Biochem. Eng. J. 2020, 161, 107666. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; El-Feky, A.M.; Ibrahim, N.E.; Sahu, R.K.; El-Sayed, A.E.K.B. Evaluation of the pancreatoprotective effect of nannochloropsis oculata extract against streptozotocin-induced diabetes in rats. J. Appl. Pharm. Sci. 2018, 8, 46–58. [Google Scholar]
- Nasirian, F.; Sarir, H.; Moradi-kor, N. Self-dual Leonard pairs antihyperglycemic and antihyperlipidemic in streptozotocin-induced diabetic rats. BioMol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Buchholz, T.; Melzig, M.F. Medicinal plants traditionally used for treatment of obesity and diabetes mellitus—Screening for pancreatic lipase and α -amylase inhibition. Phyther. Res. 2016, 30, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Rajan, L.; Palaniswamy, D.; Mohankumar, S.K. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacol. Res. 2020, 155, 104681. [Google Scholar] [CrossRef]
- Marrelli, M.; Loizzo, M.R.; Nicoletti, M.; Menichini, F. Inhibition of key enzymes linked to obesity by preparations from mediterranean dietary plants: Effects on α-amylase and pancreatic lipase activities. Plant Foods Hum. Nutr. 2013, 68, 340–346. [Google Scholar] [CrossRef]
- Birari, R.B.; Bhutani, K.K. Pancreatic lipase inhibitors from natural sources: Unexplored potential. Drug Discov. Today 2007, 12, 879–889. [Google Scholar] [CrossRef]
- Cherng, J.; Shih, M. Preventing dyslipidemia by Chlorella pyrenoidosa in rats and hamsters after chronic high fat diet treatment. Life Sci. 2005, 76, 3001–3013. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, J.; Mao, X.; Qi, P.; Zhang, X. Separation and lipid inhibition effects of a novel decapeptide from Chlorella pyenoidose. Molecules 2019, 24, 3527. [Google Scholar] [CrossRef] [Green Version]
- Moradi, S.; Ziaei, R.; Foshati, S.; Mohammadi, H.; Mostafa, S. Complementary therapies in medicine effects of spirulina supplementation on obesity: A systematic review and meta-analysis of randomized clinical trials. Complement. Ther. Med. 2019, 47, 102211. [Google Scholar] [CrossRef] [PubMed]
- Di Nicolantonio, J.J.; Bhat, A.G.; Okeefe, J. Effects of spirulina on weight loss and blood lipids: A review. Open Heart 2020, 7, 1–7. [Google Scholar]
- Fuentes-Grunewald, C.; Bayliss, C.; Zanain, M.; Pooley, C.; Scolamacchia, M.; Silkina, A. Evaluation of batch and semi-continuous culture of Porphyridium purpureum in a photobioreactor in high latitudes using Fourier Transform Infrared spectroscopy for monitoring biomass composition and metabolites production. Bioresour. Technol. 2015, 189, 357–363. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P. Profile of phenolic compounds of Prunus armeniaca L. leaf extract determined by LC-ESI-QTOF-MS/MS and their antioxidant, anti-diabetic, anti-cholinesterase, and anti-inflammatory potency. Antioxidants 2021, 10, 1869. [Google Scholar] [CrossRef]
- Wojdyło, A.; Nowicka, P.; Turkiewicz, I.P.; Tkacz, K. Profiling of polyphenols by LC-QTOF/ESI-MS, characteristics of nutritional compounds and in vitro effect on pancreatic lipase, α-glucosidase, α-amylase, cholinesterase and cyclooxygenase activities of sweet (Prunus avium) and sour (P. cerasus) cherries leaves and fruits. Ind. Crops Prod. 2021, 174, 114214. [Google Scholar]
- Wojdyło, A.; Nowicka, P.; Tkacz, K.; Turkiewicz, I.P. Fruit tree leaves as unconventional and valuable source of chlorophyll and carotenoid compounds determined by liquid chromatography-photodiode-quadrupole/time of flight-electrospray ionization-mass spectrometry (LC-PDA-qTof-ESI-MS). Food Chem. 2021, 349, 129156. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.; Hampsch-Woodill, M.; Prior, R.L. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J. Agric. Food Chem. 2001, 49, 4619–4626. [Google Scholar] [CrossRef] [PubMed]

| Microalgae Species | Flavan-3-ols | Phenolic Acids | Flavonols | Total Phenolic Content |
|---|---|---|---|---|
| P. purpureum | 207.3 ± 14.72 a | nd | nd | 207.30 ± 14.72 a |
| C. vulgaris | 114.32 ± 6.11 b,c | nd | nd | 114.32 ± 6.11 b |
| A. platensis | 49.65 ± 4.18 c | 91.49 ± 7.36 a | 1.7 ± 0.14 | 142.84 ± 11.63 a,b |
| N. oculata | 174.65 ± 44.54 a,b | 22.08 ± 5.73 b | nd | 196.72 ± 50.02 a,b |
| Microalgae Species | Total Chlorophyll Content | Total Carotenoid Content |
|---|---|---|
| P. purpureum | 0.29 ± 0.02 a | 11.35 ± 2.62 a |
| C. vulgaris | 0.39 ± 0.06 a,c | 2.03 ± 0.53 b |
| A. platensis | 0.68 ± 0.03 b | 1.16 ± 0.11 b |
| N. oculata | 0.45 ± 0.01 c | 8.60 ± 0.33 a |
| Triterpenoids | P. purpureum | C. vulgaris | A. platensis | N. oculata |
|---|---|---|---|---|
| Tormentic Acid | nd | 0.30 ± 0.00 | 4.46 ± 1.99 | 1.55 ± 0.08 |
| Alphitolic Acid | 4.34 ± 0.69 | 0.44 ± 0.03 | 30.16 ± 5.19 | 4.77 ± 2.19 |
| Maslinic Acid | 0.99 ± 0.17 | 0.47 ± 0.02 | 17.70 ± 1.70 | 1.69 ± 0.64 |
| Pomolic Acid | 1.21 ± 0.10 | 0.76 ± 0.05 | 6.12 ± 0.44 | 1.27 ± 0.40 |
| Corosolic Acid | 1.68 ± 0.01 | 0.35 ± 0.02 | 13.68 ± 4.32 | 2.08 ± 0.41 |
| Betulinic Acid | 0.58 ± 0.25 | 0.15 ± 0.01 | 2.27 ± 2.04 | 1.05 ± 0.05 |
| Oleanolic Acid | 1.12 ± 0.80 | 0.37 ± 0.03 | 40.94 ± 11.33 | 7.17 ± 0.48 |
| Ursolic Acid | 2.06 ± 1.81 | 0.51 ± 0.06 | 59.74 ± 8.56 | 8.18 ± 4.96 |
| Betulin | 0.15 ± 0.03 | 1.95 ± 0.09 | 1.71 ± 0.61 | 1.20 ± 0.81 |
| Erythrodiol | 0.04 ± 0.02 | 0.14 ± 0.00 | 4.14 ± 1.15 | 0.21 ± 0.01 |
| α-Boswellic Acid | 0.04 ± 0.00 | 2.81 ± 0.08 | 5.44 ± 3.04 | 0.17 ± 0.03 |
| Uvaol | 0.23 ± 0.05 | 0.27 ± 0.04 | 2.07 ± 0.84 | 0.35 ± 0.05 |
| Total Content | 12.04 ± 0.43 a | 8.24 ± 0.05 a | 185.81 ± 18.34 b | 29.69 ± 2.58 a |
| Cholinesterase Inhibition (% Inhib.) | α-Amylase Inhibition (IC50 mg/mL) | Pancreatic Lipase Inhibition (IC50 mg/mL) | ||
|---|---|---|---|---|
| AChE | BChE | |||
| P. purpureum | 40.89 ± 4.44 a | 31.68 ± 1.15 a | 7.50 ± 2.68 a | 3.26 ± 0.94 a |
| C. vulgaris | 29.03 ±3.33 b | 24.14 ± 3.00 b | 28.72 ± 8.30 b | 9.81 ± 1.37 b |
| A. platensis | 8.66 ± 0.75 c | 6.85 ± 1.56 c | 31.04 ± 5.29 b | 23.24 ± 1.15 c |
| N. oculata | 29.89 ± 2.26 b | 28.01 ±1.39 a,b | 12.69 ± 5.53 a,b | 3.38 ± 0.38 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vieira, M.V.; Turkiewicz, I.P.; Tkacz, K.; Fuentes-Grünewald, C.; Pastrana, L.M.; Fuciños, P.; Wojdyło, A.; Nowicka, P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules 2021, 26, 7593. https://doi.org/10.3390/molecules26247593
Vieira MV, Turkiewicz IP, Tkacz K, Fuentes-Grünewald C, Pastrana LM, Fuciños P, Wojdyło A, Nowicka P. Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules. 2021; 26(24):7593. https://doi.org/10.3390/molecules26247593
Chicago/Turabian StyleVieira, Marta Vinha, Igor Piotr Turkiewicz, Karolina Tkacz, Claudio Fuentes-Grünewald, Lorenzo M. Pastrana, Pablo Fuciños, Aneta Wojdyło, and Paulina Nowicka. 2021. "Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species" Molecules 26, no. 24: 7593. https://doi.org/10.3390/molecules26247593
APA StyleVieira, M. V., Turkiewicz, I. P., Tkacz, K., Fuentes-Grünewald, C., Pastrana, L. M., Fuciños, P., Wojdyło, A., & Nowicka, P. (2021). Microalgae as a Potential Functional Ingredient: Evaluation of the Phytochemical Profile, Antioxidant Activity and In-Vitro Enzymatic Inhibitory Effect of Different Species. Molecules, 26(24), 7593. https://doi.org/10.3390/molecules26247593