Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice
Abstract
:1. Introduction
1.1. The Plant phytocomplex
1.2. Overview on Gynostemma pentaphyllum
2. Materials and Methods
2.1. Plant Material
2.2. UHPLC-QTOF Analysis of Bioactive Compounds
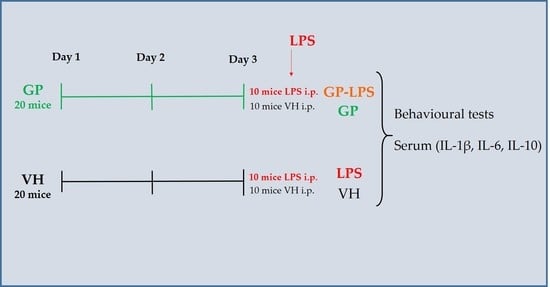
2.3. Animals and Treatment
2.4. Behavioural Test: Pole Test and Rotarod Test
2.5. ELISA Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Chemical Components in GP Extracts
3.1.1. Saponins
3.1.2. Polyphenols
3.1.3. Phytosterols
3.2. GP Restores Motor Performance after Treatment with LPS
3.2.1. Pole Test
3.2.2. Rotarod Test
3.3. GP Decreases the Inflammatory Cytokines after Treatment with LPS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Memo, M.; Mastinu, A. Plant behaviour: An evolutionary response to the environment? Plant Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Premoli, M.; Aria, F.; Bonini, S.A.; Maccarinelli, G.; Gianoncelli, A.; Memo, M.; Mastinu, A. Cannabimimetic plants: Are they new cannabinoidergic modulators? Planta 2019, 249, 1681–1694. [Google Scholar] [CrossRef] [PubMed]
- Rad, S.V.; Valadabadi, S.A.R.; Pouryousef, M.; Saifzadeh, S.; Zakrin, H.R.; Mastinu, A. Quantitative and Qualitative Evaluation of Sorghum bicolor L. under Intercropping with Legumes and Different Weed Control Methods. Horticulturae 2020, 6, 78. [Google Scholar] [CrossRef]
- Mastinu, A.; Kumar, A.; Maccarinelli, G.; Bonini, S.A.; Premoli, M.; Aria, F.; Gianoncelli, A.; Memo, M. Zeolite Clinoptilolite: Therapeutic Virtues of an Ancient Mineral. Molecules 2019, 24, 1517. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Bonini, S.A.; Rungratanawanich, W.; Aria, F.; Marziano, M.; Maccarinelli, G.; Abate, G.; Premoli, M.; Memo, M.; Uberti, D. Gamma-oryzanol Prevents LPS-induced Brain Inflammation and Cognitive Impairment in Adult Mice. Nutrients 2019, 11, 728. [Google Scholar] [CrossRef] [Green Version]
- Mastinu, A.; Premoli, M.; Ferrari-Toninelli, G.; Tambaro, S.; Maccarinelli, G.; Memo, M.; Bonini, S.A. Cannabinoids in health and disease: Pharmacological potential in metabolic syndrome and neuroinflammation. Horm. Mol. Biol. Clin. Investig. 2018, 36. [Google Scholar] [CrossRef]
- Bonini, S.A.; Premoli, M.; Tambaro, S.; Kumar, A.; Maccarinelli, G.; Memo, M.; Mastinu, A. Cannabis sativa: A comprehensive ethnopharmacological review of a medicinal plant with a long history. J. Ethnopharmacol. 2018, 227, 300–315. [Google Scholar] [CrossRef]
- Rasoanaivo, P.; Wright, C.W.; Willcox, M.L.; Gilbert, B. Whole plant extracts versus single compounds for the treatment of malaria: Synergy and positive interactions. Malar. J. 2011, 10, S4. [Google Scholar] [CrossRef] [Green Version]
- Olivés, J.; Mestres, J. Closing the Gap Between Therapeutic Use and Mode of Action in Remedial Herbs. Front. Pharmacol. 2019, 10, 1132. [Google Scholar] [CrossRef] [Green Version]
- Donno, D.; Mellano, M.G.; Cerutti, A.K.; Beccaro, G.L. Biomolecules and Natural Medicine Preparations: Analysis of New Sources of Bioactive Compounds from Ribes and Rubus spp. Buds. Pharmaceuticals 2016, 9, 7. [Google Scholar] [CrossRef]
- Fodaroni, G.; Burico, M.; Gaetano, A.; Maidecchi, A.; Pagiotti, R.; Mattoli, L.; Traldi, P.; Ragazzi, E. An integrated approach to the evaluation of a metabolomic fingerprint for a phytocomplex. Focus on artichoke (Cynara cardunculus subsp. Scolymus) leaf. Nat. Prod. Commun. 2014, 9, 565–568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdavi, A.; Moradi, P.; Mastinu, A. Variation in Terpene Profiles of Thymus vulgaris in Water Deficit Stress Response. Molecules 2020, 25, 1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, S.; Griffin, T.S.; Kraner, D.; Schaffner, M.K.; Sharma, D.; Hazel, M.; Leitch, A.R.; Orians, C.M.; Han, W.; Stepp, J.R.; et al. Environmental Factors Variably Impact Tea Secondary Metabolites in the Context of Climate Change. Front. Plant Sci. 2019, 10, 939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reza Yousefi, A.; Rashidi, S.; Moradi, P.; Mastinu, A. Germination and Seedling Growth Responses of Zygophyllum fabago, Salsola kali L. and Atriplex canescens to PEG-Induced Drought Stress. Environments 2020, 7, 107. [Google Scholar] [CrossRef]
- Buriani, A.; Fortinguerra, S.; Sorrenti, V.; Caudullo, G.; Carrara, M. Essential Oil Phytocomplex Activity, a Review with a Focus on Multivariate Analysis for a Network Pharmacology-Informed Phytogenomic Approach. Molecules 2020, 25, 1833. [Google Scholar] [CrossRef] [Green Version]
- Ettorre, A.; Frosali, S.; Andreassi, M.; Di Stefano, A. Lycopene phytocomplex, but not pure lycopene, is able to trigger apoptosis and improve the efficacy of photodynamic therapy in HL60 human leukemia cells. Exp. Biol. Med. 2010, 235, 1114–1125. [Google Scholar] [CrossRef]
- Babaskin, D.V.; Litvinova, T.M.; Babaskina, L.I. The Effect of the Phytocomplex Electrophoresis on the Clinical Symptomatology and Quality of Life of Patients with the Knee Joint Osteoarthritis. Open Access Maced. J. Med. Sci. 2019, 7, 2236–2241. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Su, H.; Yang, J.; Feng, L.; Li, Z.; Zhao, G. Population genetic structure, migration, and polyploidy origin of a medicinal species Gynostemma pentaphyllum (Cucurbitaceae). Methods Ecol. Evol. 2019, 9, 11145–11170. [Google Scholar] [CrossRef] [Green Version]
- Abid, S.; Mohanan, P.; Kaliraj, L.; Park, J.K.; Ahn, J.C.; Yang, D.C. Development of species-specific chloroplast markers for the authentication of Gynostemma pentaphyllum and their distribution in the Korean peninsula. Fitoterapia 2019, 138, 104295. [Google Scholar] [CrossRef]
- Wang, B.; Li, M.; Gao, H.; Sun, X.; Gao, B.; Zhang, Y.; Yu, L. Chemical composition of tetraploid Gynostemma pentaphyllum gypenosides and their suppression on inflammatory response by NF-kappaB/MAPKs/AP-1 signaling pathways. Food Sci. Nutr. 2020, 8, 1197–1207. [Google Scholar] [CrossRef]
- Lee, H.S.; Lim, S.M.; Jung, J.I.; Kim, S.M.; Lee, J.K.; Kim, Y.H.; Cha, K.M.; Oh, T.K.; Moon, J.M.; Kim, T.Y.; et al. Gynostemma Pentaphyllum Extract Ameliorates High-Fat Diet-Induced Obesity in C57BL/6N Mice by Upregulating SIRT1. Nutrients 2019, 11, 2475. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, K.; Du, Y.; Fan, Q.; Tang, C.Y.; He, J.F. Gypenosides might have neuroprotective and immunomodulatory effects on optic neuritis. Med. Hypotheses 2014, 82, 636–638. [Google Scholar] [CrossRef] [PubMed]
- Wong, W.Y.; Lee, M.M.; Chan, B.D.; Ma, V.W.; Zhang, W.; Yip, T.T.; Wong, W.T.; Tai, W.C. Gynostemma pentaphyllum saponins attenuate inflammation in vitro and in vivo by inhibition of NF-kappaB and STAT3 signaling. Oncotarget 2017, 8, 87401–87414. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Ma, C.; Li, H.; Dev, S.; He, J.; Qu, X. Medicinal Value and Potential Therapeutic Mechanisms of Gynostemma pentaphyllum (Thunb.) Makino and Its Derivatives: An Overview. Curr. Top. Med. Chem. 2019, 19, 2855–2867. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Cai, Z.; Song, L.; Liu, Y.; Wang, Q.; Feng, X. Gynostemma pentaphyllum Attenuates the Progression of Nonalcoholic Fatty Liver Disease in Mice: A Biomedical Investigation Integrated with In Silico Assay. Evid. Based Complement. Altern. Med. 2018, 2018, 8384631. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.; Shi, L.; Qi, G.; Zhao, S.; Gao, Y.; Li, Y. Gypenoside Protects Cardiomyocytes against Ischemia-Reperfusion Injury via the Inhibition of Mitogen-Activated Protein Kinase Mediated Nuclear Factor Kappa B Pathway In Vitro and In Vivo. Front. Pharmacol. 2016, 7, 148. [Google Scholar] [CrossRef] [Green Version]
- Zhao, J.; Bi, W.; Xiao, S.; Lan, X.; Cheng, X.; Zhang, J.; Lu, D.; Wei, W.; Wang, Y.; Li, H.; et al. Neuroinflammation induced by lipopolysaccharide causes cognitive impairment in mice. Sci. Rep. 2019, 9, 5790. [Google Scholar] [CrossRef] [Green Version]
- Rocchetti, G.; Lucini, L.; Chiodelli, G.; Giuberti, G.; Gallo, A.; Masoero, F.; Trevisan, M. Phenolic profile and fermentation patterns of different commercial gluten-free pasta during in vitro large intestine fermentation. Food Res. Int. 2017, 97, 78–86. [Google Scholar] [CrossRef]
- Rocchetti, G.; Tomas, M.; Zhang, L.; Zengin, G.; Lucini, L.; Capanoglu, E. Red beet (Beta vulgaris) and amaranth (Amaranthus sp.) microgreens: Effect of storage and in vitro gastrointestinal digestion on the untargeted metabolomic profile. Food Res. Int. 2020, 332, 127415. [Google Scholar] [CrossRef]
- Zhang, L.; Rocchetti, G.; Zengin, G.; Ak, G.; Yıldıztugay, E.; Mahomoodally, M.F.; Picot-Allain, M.C.N.; Lucini, L. Profiling of polyphenols and sesquiterpenoids using different extraction methods in Muscari turcicum, an endemic plant from Turkey. Ind. Crops Prod. 2020, 154, 112626. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’Hiri, N.; Garcia-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S.; et al. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef] [PubMed]
- Rocchetti, G.; Lucini, L.; Rodriguez, J.M.L.; Barba, F.J.; Giuberti, G. Gluten-free flours from cereals, pseudocereals and legumes: Phenolic fingerprints and in vitro antioxidant properties. Food Chem. 2019, 271, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Seemann, S.; Zohles, F.; Lupp, A. Comprehensive comparison of three different animal models for systemic inflammation. J. Biomed. Sci. 2017, 24. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Chesselet, M.-F. Techniques for Motor Assessment in Rodents. Mov. Disord. 2015, 139–157. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, Y.; Liu, B.; Sun, X.; Li, Z.; Chen, Y.; Guo, Z.; Liu, H.; Li, D.; Wang, C.; Zhu, X.; et al. Protective effects of alfalfa saponins on oxidative stress-induced apoptotic cells. Food Funct. 2020, 11, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Juang, Y.P.; Liang, P.H. Biological and Pharmacological Effects of Synthetic Saponins. Molecules 2020, 25, 4974. [Google Scholar] [CrossRef]
- Sun, J.; Yu, X.; Huangpu, H.; Yao, F. Ginsenoside Rb3 protects cardiomyocytes against hypoxia/reoxygenation injury via activating the antioxidation signaling pathway of PERK/Nrf2/HMOX1. Biomed. Pharmacother. 2019, 109, 254–261. [Google Scholar] [CrossRef]
- Razmovski-Naumovski, V.; Huang, T.H.-W.; Tran, V.H.; Li, G.Q.; Duke, C.C.; Roufogalis, B.D. Chemistry and Pharmacology of Gynostemma pentaphyllum. Phytochem. Rev. 2005, 4, 197–219. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, P.; Shin, C.Y. A comprehensive review of the therapeutic and pharmacological effects of ginseng and ginsenosides in central nervous system. J. Ginseng Res. 2013, 37, 8–29. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.K.; Ye, Y.; Zhao, Z.N.; Li, K.J.; Du, Y.; Hu, Q.M.; He, J.F. Neuroprotective effects of gypenosides in experimental autoimmune optic neuritis. Int. J. Ophthalmol. 2017, 10, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.H.; Fang, X.Y.; Wang, S.S.; Li, C.F.; Chen, S.M.; Chen, X.M.; Liu, Q.; Li, Y.C.; Yi, L.T. Antidepressant-like effects of standardized gypenosides: Involvement of brain-derived neurotrophic factor signaling in hippocampus. Psychopharmacology 2016, 233, 3211–3221. [Google Scholar] [CrossRef] [PubMed]
- Gou, S.H.; Huang, H.F.; Chen, X.Y.; Liu, J.; He, M.; Ma, Y.Y.; Zhao, X.N.; Zhang, Y.; Ni, J.M. Lipid-lowering, hepatoprotective, and atheroprotective effects of the mixture Hong-Qu and gypenosides in hyperlipidemia with NAFLD rats. J. Chin. Med. Assoc. 2016, 79, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Banez, M.J.; Geluz, M.I.; Chandra, A.; Hamdan, T.; Biswas, O.S.; Bryan, N.S.; Von Schwarz, E.R. A systemic review on the antioxidant and anti-inflammatory effects of resveratrol, curcumin, and dietary nitric oxide supplementation on human cardiovascular health. Nutr. Res. 2020, 78, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Calvet, A.; Euba, B.; Caballero, L.; Diez-Martinez, R.; Menendez, M.; Ortiz de Solorzano, C.; Leiva, J.; Micol, V.; Barrajon-Catalan, E.; Garmendia, J. Preclinical Evaluation of the Antimicrobial-Immunomodulatory Dual Action of Xenohormetic Molecules against Haemophilus influenzae Respiratory Infection. Biomolecules 2019, 9, 891. [Google Scholar] [CrossRef] [Green Version]
- Rebas, E.; Rzajew, J.; Radzik, T.; Zylinska, L. Neuroprotective Polyphenols: A Modulatory Action on Neurotransmitter Pathways. Curr. Neuropharmacol. 2020, 18, 431–445. [Google Scholar] [CrossRef]
- Pogacnik, L.; Ota, A.; Ulrih, N.P. An Overview of Crucial Dietary Substances and Their Modes of Action for Prevention of Neurodegenerative Diseases. Cells 2020, 9, 576. [Google Scholar] [CrossRef] [Green Version]
- Palungwachira, P.; Tancharoen, S.; Phruksaniyom, C.; Klungsaeng, S.; Srichan, R.; Kikuchi, K.; Nararatwanchai, T. Antioxidant and Anti-Inflammatory Properties of Anthocyanins Extracted from Oryza sativa L. in Primary Dermal Fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 2089817. [Google Scholar] [CrossRef] [Green Version]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Anthocyanins, colour and antioxidant properties of eggplant (Solanum melongena L.) and violet pepper (Capsicum annuum L.) peel extracts. Z. Naturforsch. C J. Biosci. 2006, 61, 527–535. [Google Scholar] [CrossRef]
- Khan, M.S.; Ali, T.; Kim, M.W.; Jo, M.H.; Jo, M.G.; Badshah, H.; Kim, M.O. Anthocyanins protect against LPS-induced oxidative stress-mediated neuroinflammation and neurodegeneration in the adult mouse cortex. Neurochem. Int. 2016, 100, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Slovák, L.; Švík, K.; Mihalová, D.; Tóth, J.; Czigle, S.; Pašková, Ľ.; Bilka, F.; Bauerová, K. Ferulaldehyde Improves the Effect of Methotrexate in Experimental Arthritis. Molecules 2017, 22, 1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Šamec, D.; Valek-Žulj, L.; Martinez, S.; Grúz, J.; Piljac, A.; Piljac-Žegarac, J. Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind. Crops Prod. 2016, 84, 104–107. [Google Scholar] [CrossRef]
- Boulghobra, D.; Grillet, P.E.; Laguerre, M.; Tenon, M.; Fauconnier, J.; Fanca-Berthon, P.; Reboul, C.; Cazorla, O. Sinapine, but not sinapic acid, counteracts mitochondrial oxidative stress in cardiomyocytes. Redox Biol. 2020, 34, 101554. [Google Scholar] [CrossRef]
- Pathak, G.; Singh, S.; Kumari, P.; Raza, W.; Hussain, Y.; Meena, A. Cirsimaritin, a lung squamous carcinoma cells (NCIH-520) proliferation inhibitor. J. Biomol. Struct. Dyn. 2020. [Google Scholar] [CrossRef]
- Shin, M.S.; Park, J.Y.; Lee, J.; Yoo, H.H.; Hahm, D.H.; Lee, S.C.; Lee, S.; Hwang, G.S.; Jung, K.; Kang, K.S. Anti-inflammatory effects and corresponding mechanisms of cirsimaritin extracted from Cirsium japonicum var. maackii Maxim. Bioorg. Med. Chem. Lett. 2017, 27, 3076–3080. [Google Scholar] [CrossRef]
- Ren, X.; Bao, Y.; Zhu, Y.; Liu, S.; Peng, Z.; Zhang, Y.; Zhou, G. Isorhamnetin, Hispidulin, and Cirsimaritin Identified in Tamarix ramosissima Barks from Southern Xinjiang and Their Antioxidant and Antimicrobial Activities. Molecules 2019, 24, 390. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.-F.; Kobayashi, J.i.; Nakamura, H.; Ohizumi, Y.; Hirata, Y.; Sasaki, T. Penasterol, a novel antileukemic sterol from the okinawan marine sponge Penares sp. J. Chem. Soc. Perkin Trans. I 1988. [Google Scholar] [CrossRef]
- Peng, L. Sitosterol-beta -glucoside as Primer for Cellulose Synthesis in Plants. Science 2002, 295, 147–150. [Google Scholar] [CrossRef]
- Vilahur, G.; Ben-Aicha, S.; Diaz-Riera, E.; Badimon, L.; Padro, T. Phytosterols and Inflammation. Curr. Med. Chem. 2019, 26, 6724–6734. [Google Scholar] [CrossRef]
- Gabay, O.; Sanchez, C.; Salvat, C.; Chevy, F.; Breton, M.; Nourissat, G.; Wolf, C.; Jacques, C.; Berenbaum, F. Stigmasterol: A phytosterol with potential anti-osteoarthritic properties. Osteoarthr. Cartil. 2010, 18, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Torvell, M.; Hampton, D.W.; Connick, P.; MacLullich, A.M.J.; Cunningham, C.; Chandran, S. A single systemic inflammatory insult causes acute motor deficits and accelerates disease progression in a mouse model of human tauopathy. Alzheimers Dement. 2019, 5, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Krzyszton, C.P.; Sparkman, N.L.; Grant, R.W.; Buchanan, J.B.; Broussard, S.R.; Woods, J.; Johnson, R.W. Exacerbated fatigue and motor deficits in interleukin-10-deficient mice after peripheral immune stimulation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1109–R1114. [Google Scholar] [CrossRef] [PubMed]
- Bonini, S.A.; Mastinu, A.; Maccarinelli, G.; Mitola, S.; Premoli, M.; La Rosa, L.R.; Ferrari-Toninelli, G.; Grilli, M.; Memo, M. Cortical Structure Alterations and Social Behavior Impairment in p50-Deficient Mice. Cereb. Cortex 2016, 26, 2832–2849. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Decker, J.T.; Margul, D.J.; Smith, D.R.; Cummings, B.J.; Anderson, A.J.; Shea, L.D. Local Immunomodulation with Anti-inflammatory Cytokine-Encoding Lentivirus Enhances Functional Recovery after Spinal Cord Injury. Mol. Ther. 2018, 26, 1756–1770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poulsen, N.B.; Lambert, M.N.T.; Jeppesen, P.B. The Effect of Plant Derived Bioactive Compounds on Inflammation: A Systematic Review and Meta-Analysis. Mol. Nutr. Food Res. 2020, 64, e2000473. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, P.H.; Gauhar, R.; Hwang, S.L.; Dao, T.T.; Park, D.C.; Kim, J.E.; Song, H.; Huh, T.L.; Oh, W.K. New dammarane-type glucosides as potential activators of AMP-activated protein kinase (AMPK) from Gynostemma pentaphyllum. Bioorg. Med. Chem. 2011, 19, 6254–6260. [Google Scholar] [CrossRef]
- Turner, M.D.; Nedjai, B.; Hurst, T.; Pennington, D.J. Cytokines and chemokines: At the crossroads of cell signalling and inflammatory disease. Biochim. Biophys. Acta 2014, 1843, 2563–2582. [Google Scholar] [CrossRef] [Green Version]
- Bailly, S.; Ferrua, B.; Fay, M.; Gougerot-Pocidalo, M.A. Differential regulation of IL 6, IL 1 A, IL 1 beta and TNF alpha production in LPS-stimulated human monocytes: Role of cyclic AMP. Cytokine 1990, 2, 205–210. [Google Scholar] [CrossRef]
- de Bont, N.; Netea, M.G.; Rovers, C.; Smilde, T.; Hijmans, A.; Demacker, P.N.; van der Meer, J.W.; Stalenhoef, A.F. LPS-induced release of IL-1 beta, IL-1Ra, IL-6, and TNF-alpha in whole blood from patients with familial hypercholesterolemia: No effect of cholesterol-lowering treatment. J. Interferon Cytokine Res. 2006, 26, 101–107. [Google Scholar] [CrossRef]
- Eggesbo, J.B.; Hjermann, I.; Hostmark, A.T.; Kierulf, P. LPS induced release of IL-1 beta, IL-6, IL-8 and TNF-alpha in EDTA or heparin anticoagulated whole blood from persons with high or low levels of serum HDL. Cytokine 1996, 8, 152–160. [Google Scholar] [CrossRef] [PubMed]
- During, A.; Debouche, C.; Raas, T.; Larondelle, Y. Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells. J. Nutr. 2012, 142, 1798–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.J.; Park, C.G.; Varghese, R.; Lee, J.Y.; Kim, Y.; Sung, G.H. In-vitro antioxidative, antiinflammatory properties of Aurea helianthus leaf extract a Korean traditional medicinal plant. Saudi J. Biol. Sci. 2017, 24, 1943–1947. [Google Scholar] [CrossRef] [PubMed]
- Miraghazadeh, S.G.; Shafaroodi, H.; Asgarpanah, J. Analgesic and antiinflammatory activities of the essential oil of the unique plant Zhumeria majdae. Nat. Prod. Commun. 2015, 10, 669–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, S.-Y.; Zhou, S.-F.; Gao, S.-H.; Yu, Z.-L.; Zhang, S.-F.; Tang, M.-K.; Sun, J.-N.; Ma, D.-L.; Han, Y.-F.; Fong, W.-F.; et al. New Perspectives on How to Discover Drugs from Herbal Medicines: CAM’s Outstanding Contribution to Modern Therapeutics. Evid. Based Complement. Altern. Med. 2013, 2013, 1–25. [Google Scholar] [CrossRef] [Green Version]






| Name | Formula | mg/L ± SD (as Ginsenoside-Rb eq.) |
|---|---|---|
| Ginsenoside “RF1”/“RF2”/“RF2-2” | C42 H72 O13 | 4.67 ± 0.23 |
| Protopanaxatriols | C30 H52 O4 | 1.66 ± 0.10 |
| Ginsenoside RH2 | C36 H62 O8 | 1.44 ± 0.05 |
| Protopanaxadiols | C30 H52 O3 | 1.27 ± 0.02 |
| Ginsenoside RD | C48 H82 O18 | 1.07 ± 0.02 |
| Gypenoside XXV | C47 H78 O18 | 1.06 ± 0.02 |
| Ginsenoside RF | C42 H72 O14 | 0.68 ± 0.01 |
| Isofucosterolo | C29 H48 O | 0.52 ± 0.01 |
| Ginsenoside RB1 | C52 H92 O23 | 0.36 ± 0.02 |
| Gypenoside XLVI | C48 H82 O19 | 0.31 ± 0.05 |
| Phyllodulcin | C16 H14 O5 | 0.09 ± 0.01 |
| Name | Formula | mg/L ± SD (as cholesterol eq.) |
|---|---|---|
| 4,4-dimethyl-14-carboxy-cholesta-9(10),24-dien-3beta-ol | C30 H48 O3 | 178.19 ± 14.04 |
| 3-O-(6′-O-(7Z,10Z-hexadecadienoyl)-beta-d-glucopyranosyl)-stigmast-5-en-3beta-ol | C51 H86 O7 | 62.35 ± 2.08 |
| (22R)-22-hydroxystigmast-4-en-3-one | C29 H48 O2 | 53.60 ± 7.39 |
| (22S)-1alpha-acetoxy-5alpha-furospirostan-3alpha,11beta,20R-triol | C30 H48 O7 | 36.04 ± 2.11 |
| 3beta-hydroxy-4alpha-methyl-5alpha-cholest-7-ene-4beta-carboxylic acid | C29 H48 O3 | 32.70 ± 6.02 |
| 4-methylene-5alpha-poriferast-8(14)-en-3beta,7alpha,15beta-triol | C30 H50 O3 | 30.20 ± 0.95 |
| 3beta-acetoxy-cholest-5-en-7-one | C29 H46 O3 | 29.17 ± 5.98 |
| 4-methylene-5alpha-poriferast-8(9)-en-3beta,11beta,14alpha,15alpha-tetrol | C30 H50 O4 | 26.13 ± 1.02 |
| 24-isopropenyl-cholesta-5,22E-dien-3beta-ol | C30 H48 O | 24.38 ± 4.66 |
| Other phytosterols | 857.00 ± 49.67 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mastinu, A.; Bonini, S.A.; Premoli, M.; Maccarinelli, G.; Mac Sweeney, E.; Zhang, L.; Lucini, L.; Memo, M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules 2021, 26, 570. https://doi.org/10.3390/molecules26030570
Mastinu A, Bonini SA, Premoli M, Maccarinelli G, Mac Sweeney E, Zhang L, Lucini L, Memo M. Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules. 2021; 26(3):570. https://doi.org/10.3390/molecules26030570
Chicago/Turabian StyleMastinu, Andrea, Sara Anna Bonini, Marika Premoli, Giuseppina Maccarinelli, Eileen Mac Sweeney, Leilei Zhang, Luigi Lucini, and Maurizio Memo. 2021. "Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice" Molecules 26, no. 3: 570. https://doi.org/10.3390/molecules26030570
APA StyleMastinu, A., Bonini, S. A., Premoli, M., Maccarinelli, G., Mac Sweeney, E., Zhang, L., Lucini, L., & Memo, M. (2021). Protective Effects of Gynostemma pentaphyllum (var. Ginpent) against Lipopolysaccharide-Induced Inflammation and Motor Alteration in Mice. Molecules, 26(3), 570. https://doi.org/10.3390/molecules26030570