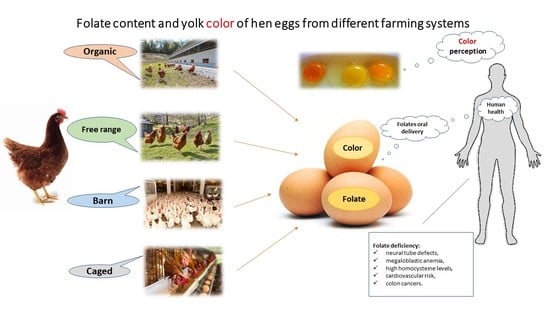
Folate Content and Yolk Color of Hen Eggs from Different Farming Systems
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Samples
3.2. Chemicals, Enzymes, and Standards
3.3. Sample Preparation
3.4. Folate Quantification
3.5. Egg Yolk Color Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Blancquaert, D.; De Steur, H.; Gellynck, X.; Van Der Straeten, D. Present and future of folate biofortification of crop plants. J. Exp. Bot. 2014, 65, 895–906. [Google Scholar] [CrossRef] [Green Version]
- Delchier, N.; Ringling, C.; Maingonnat, J.-F.; Rychlik, M.; Renard, C.M. Mechanisms of folate losses during processing: Diffusion vs. heat degradation. Food Chem. 2014, 157, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Delchier, N.; Ringling, C.; Le Grandois, J.; Aoudé-Werner, D.; Galland, R.; Georgé, S.; Rychlik, M.; Renard, C.M. Effects of industrial processing on folate content in green vegetables. Food Chem. 2013, 139, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Dang, J. Folate retention in selected processed legumes. Food Chem. 2000, 68, 295–298. [Google Scholar] [CrossRef]
- Fajardo, V.; Alonso-Aperte, E.; Varela-Moreiras, G. Total folate content in ready-to-eat vegetable meals from the Spanish market. J. Food Compos. Anal. 2017, 64, 223–231. [Google Scholar] [CrossRef]
- Fajardo, V.; Alonso-Aperte, E.; Varela-Moreiras, G. Lack of data on folate in convenience foods: Should ready-to-eat products be considered relevant for folate intake? The European challenge. J. Food Compos. Anal. 2012, 28, 155–163. [Google Scholar] [CrossRef]
- Delchier, N.; Herbig, A.-L.; Rychlik, M.; Renard, C.M. Folates in Fruits and Vegetables: Contents, Processing, and Stability. Compr. Rev. Food Sci. Food Saf. 2016, 15, 506–528. [Google Scholar] [CrossRef] [Green Version]
- López-Nicolás, R.; Frontela-Saseta, C.; González-Abellán, R.; Barado-Piqueras, A.; Perez-Conesa, D.; Ros, G. Folate fortification of white and whole-grain bread by adding Swiss chard and spinach. Acceptability by consumers. LWT 2014, 59, 263–269. [Google Scholar] [CrossRef]
- Huo, Y.; Qin, X.; Wang, J.; Sun, N.; Zeng, Q.; Liu, L.; Xu, X.; Wang, X. Efficacy of folic acid supplementation in stroke prevention: New insight from a meta-analysis. Int. J. Clin. Pr. 2012, 66, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Eichholzer, M.; Tönz, O.; Zimmermann, R. Folic acid: A public-health challenge. Lancet 2006, 367, 1352–1361. [Google Scholar] [CrossRef]
- Kim, Y.-I. Folate and colorectal cancer: An evidence-based critical review. Mol. Nutr. Food Res. 2007, 51, 267–292. [Google Scholar] [CrossRef]
- Lee, J.E.; Chan, A.T. Fruit, Vegetables, and Folate: Cultivating the Evidence for Cancer Prevention. Gastroenterology 2011, 141, 16–20. [Google Scholar] [CrossRef] [Green Version]
- Morris, M.S.; Jacques, P.F.; Rosenberg, I.H.; Selhub, J. Folate and vitamin B-12 status in relation to anemia, macrocytosis, and cognitive impairment in older Americans in the age of folic acid fortification. Am. J. Clin. Nutr. 2007, 85, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, S.; Virtanen, J.K.; Rissanen, T.H.; Alfthan, G.; Laukkanen, J.; Nyyssönen, K.; Mursu, J.; Valkonen, V.-P.; Tuomainen, T.-P.; Kaplan, G.A.; et al. Serum folate and homocysteine and the incidence of acute coronary events: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2004, 80, 317–323. [Google Scholar] [CrossRef] [Green Version]
- Virtanen, J.K.; Voutilainen, S.; Happonen, P.; Alfthan, G.; Kaikkonen, J.; Mursu, J.; Rissanen, T.H.; Kaplan, G.A.; Korhonen, M.J.; Sivenius, J.; et al. Serum homocysteine, folate and risk of stroke: Kuopio Ischaemic Heart Disease Risk Factor (KIHD) Study. Eur. J. Cardiovasc. Prev. Rehabil. 2005, 12, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Arth, A.; Kancherla, V.; Pachón, H.; Zimmerman, S.; Johnson, Q.; Oakley, G.P. A 2015 global update on folic acid-preventable spina bifida and anencephaly. Birth Defects Res. Part A Clin. Mol. Teratol. 2016, 106, 520–529. [Google Scholar] [CrossRef] [PubMed]
- Institute of Medicine Dietary Reference Intakes. In Dietary Reference Intakes; National Academies Press: Washington, DC, USA, 2003.
- Kelly, P.; McPartlin, J.; Goggins, M.; Weir, D.G.; Scott, J.M. Unmetabolized folic acid in serum: Acute studies in subjects consuming fortified food and supplements. Am. J. Clin. Nutr. 1997, 65, 1790–1795. [Google Scholar] [CrossRef]
- Sweeney, M.R.; McPartlin, J.; Scott, J. Folic acid fortification and public health: Report on threshold doses above which unmetabolised folic acid appear in serum. BMC Public Health 2007, 7, 41. [Google Scholar] [CrossRef] [Green Version]
- Savage, D.G.; Lindenbaum, J. Folate-cobalamin interactions. In Folate in Health and Disease, 1st ed.; Bailey, L.B., Ed.; Marcel Dekker Inc.: New York, NY, USA, 1995; pp. 237–285. [Google Scholar]
- Altic, L.; McNulty, H.; Hoey, L.; McAnena, L.; Pentieva, K. Validation of Folate-Enriched Eggs as a Functional Food for Improving Folate Intake in Consumers. Nutrition 2016, 8, 777. [Google Scholar] [CrossRef] [Green Version]
- Vahteristo, L.; Lehikoinen, K.; Ollilainen, V.; Varo, P. Application of an HPLC assay for the determination of folate derivatives in some vegetables, fruits and berries consumed in Finland. Food Chem. 1997, 59, 589–597. [Google Scholar] [CrossRef]
- Hefni, M.; Witthöft, C.M. Folate content in processed legume foods commonly consumed in Egypt. LWT 2014, 57, 337–343. [Google Scholar] [CrossRef]
- Maharaj, P.P.; Prasad, S.; Devi, R.; Gopalan, R. Folate content and retention in commonly consumed vegetables in the South Pacific. Food Chem. 2015, 182, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Gujska, E.; Michalak, J.; Klepacka, J. Folates Stability in Two Types of Rye Breads During Processing and Frozen Storage. Plant Foods Hum. Nutr. 2009, 64, 129–134. [Google Scholar] [CrossRef]
- Striegel, L.; Weber, N.; Dumler, C.; Chebib, S.; Netzel, M.E.; Sultanbawa, Y.; Rychlik, M. Promising Tropical Fruits High in Folates. Foods 2019, 8, 363. [Google Scholar] [CrossRef] [Green Version]
- Harman, N.L.; Leeds, A.R.; Griffin, B.A. Increased dietary cholesterol does not increase plasma low density lipoprotein when accompanied by an energy-restricted diet and weight loss. Eur. J. Nutr. 2008, 47, 287–293. [Google Scholar] [CrossRef]
- Omri, B.; Alloui, N.; Durazzo, A.; Lucarini, M.; Aiello, A.; Romano, R.; Santini, A.; Abdouli, H. Egg Yolk Antioxidants Profiles: Effect of Diet Supplementation with Linseeds and Tomato-Red Pepper Mixture before and after Storage. Foods 2019, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nys, Y.; Sauveur, B. Valeur nutritionnelle des oeufs. INRA Prod. Anim. 2004, 17, 385–393. [Google Scholar] [CrossRef]
- Drewnowski, A. The Nutrient Rich Foods Index helps to identify healthy, affordable foods. Am. J. Clin. Nutr. 2010, 91, 1095S–1101S. [Google Scholar] [CrossRef] [PubMed]
- Réhault-Godbert, S.; Guyot, N.; Nys, Y. The Golden Egg: Nutritional Value, Bioactivities, and Emerging Benefits for Human Health. Nutrition 2019, 11, 684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnowska-Kujawska, M.; Gujska, E.; Michalak, J. Folate determination in livers of different animal species. Czech J. Food Sci. 2020, 38, 43–48. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Draszanowska, A.; Gujska, E. Effect of Different Cooking Methods on Folate Content in Chicken Liver. Foods 2020, 9, 1431. [Google Scholar] [CrossRef] [PubMed]
- Strandler, H.S.; Jastrebova, J.; Mattisson, I. Folate content in Swedish eggs: Influence of breed, feed and processing. Eur. Food Res. Technol. 2011, 233, 923–930. [Google Scholar] [CrossRef]
- Żakowska-Biemans, S.; Tekień, A. Free Range, Organic? Polish Consumers Preferences Regarding Information on Farming System and Nutritional Enhancement of Eggs: A Discrete Choice Based Experiment. Sustainability 2017, 9, 1999. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, D.; Kallas, Z.; Pappa, M.; Gil, J.M. Are Consumers’ Egg Preferences Influenced by Animal-Welfare Conditions and Environmental Impacts? Sustainability 2019, 11, 6218. [Google Scholar] [CrossRef] [Green Version]
- Goddard, E.; Boxall, P.; Emunu, J.P.; Boyd, C.; Asselin, A.; Neall, A. Consumer Attitudes, Willingness to Pay and Revealed Preferences for Different Egg Production Attributes: Analysis of Canadian Egg Consumers; Project Report no. 07-03; De-partment of Rural Economy, University of Alberta, Edmonton: Alberta, AB, Canada, 2007; Available online: http://ageconsearch.umn.edu/bitstream/52087/2/PR%2007-03.pdf (accessed on 5 July 2019).
- Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv%3AOJ.L_.2018.150.01.0001.01.ENG (accessed on 1 December 2020).
- Sherwood, T.; Alphin, R.; Saylor, W.; White, H. Folate Metabolism and Deposition in Eggs by Laying Hens. Arch. Biochem. Biophys. 1993, 307, 66–72. [Google Scholar] [CrossRef]
- Jastrebova, J.; Strandler, H.S.; Patring, J.; Wiklund, T. Comparison of UPLC and HPLC for Analysis of Dietary Folates. Chromatograpy. 2011, 73, 219–225. [Google Scholar] [CrossRef]
- Vahteristo, L.T.; Ollilainen, V.; Varo, P. Liquid chromatographic determination of folate monoglutamates in fish, meat, egg, and dairy products consumed in Finland. J. AOAC Int. 1997, 80, 373–378. [Google Scholar] [CrossRef] [Green Version]
- Soongsongkiat, M.; Puwastien, P.; Jittinandana, S.; Dee-Uam, A.; Sungpuag, P. Testing of folate conjugase from chicken pancreas vs. commercial enzyme and studying the effect of cooking on folate retention in Thai foods. J. Food Compos. Anal. 2010, 23, 681–688. [Google Scholar] [CrossRef]
- Han, Y.H.; Yon, M.; Hyun, T.H. Folate intake estimated with an updated database and its association to blood folate and homocysteine in Korean college students. Eur. J. Clin. Nutr. 2004, 59, 246–254. [Google Scholar] [CrossRef] [Green Version]
- Kunachowicz, H.; Przygoda, B.; Nadolna, I.; Iwanow, K. Warzywa i przetwory warzywne. In Tabele składu i wartości odżywczej żywności; Kunachowicz, H., Ed.; PZWL Wydawnictwo Lekarskie: Warsaw, Poland, 2018; p. 89. [Google Scholar]
- USDA. FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 December 2020).
- Livsmedelsverket. Swedish Food Agency. Available online: http://www7.slv.se/SokNaringsinnehall/# (accessed on 1 December 2020).
- Gałązka-Czarnecka, I.; Korzeniewska, E.; Czarnecki, A.; Sójka, M.; Kiełbasa, P.; Dróżdź, T. Evaluation of Quality of Eggs from Hens Kept in Caged and Free-Range Systems Using Traditional Methods and Ultra-Weak Luminescence. Appl. Sci. 2019, 9, 2430. [Google Scholar] [CrossRef] [Green Version]
- Krawczyk, J. Effect of layer age and egg production level on changes in quality of eggs from hens of conservation breeds and commercial hybrids. Ann. Anim. Sci. 2009, 9, 185–193. [Google Scholar]
- Dudek, M.; Rabsztyn, A. Egg quality of dual-purpose hens intended for small-scale farming. Acta Sci. Pol. Zootech. 2011, 10, 3–12. [Google Scholar]
- Wang, J.; Yue, H.; Wu, S.; Zhang, H.; Qi, G. Nutritional modulation of health, egg quality and environmental pollution of the layers. Anim. Nutr. 2017, 3, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Batkowska, J.; Brodacki, A. Selected quality traits of eggs and the productivity of newly created laying hen hybrids dedicated to an extensive rearing system. Arch. Anim. Breed. 2017, 60, 87–93. [Google Scholar] [CrossRef] [Green Version]
- Şekeroǧlu, A.; Altuntaş, E. Effects of egg weight on egg quality characteristics. J. Sci. Food Agric. 2008, 89, 379–383. [Google Scholar] [CrossRef]
- Shin, D.; Narciso-Gaytán, C.; Regenstein, J.M.; Sánchez-Plata, M.X. Effect of various refrigeration temperatures on quality of shell eggs. J. Sci. Food Agric. 2011, 92, 1341–1345. [Google Scholar] [CrossRef]
- Batkowska, J.; Brodacki, A.; Knaga, S. Quality of Laying Hen Eggs During Storage Depending on Egg Weight and Type of Cage System (Conventional vs. Furnished Cages). Ann. Anim. Sci. 2014, 14, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wu, S.; Zhang, H.; Yue, H.; Qi, G.; Li, J. Effect of dietary protein sources and storage temperatures on egg internal quality of stored shell eggs. Anim. Nutr. 2015, 1, 299–304. [Google Scholar] [CrossRef]
- Czarnowska-Kujawska, M.; Gujska, E.; Michalak, J. Testing of different extraction procedures for folate HPLC determination in fresh fruits and vegetables. J. Food Compos. Anal. 2017, 57, 64–72. [Google Scholar] [CrossRef]
- Jarosz, M.; Stoś, K.; Przygoda, B.; Matczuk, E.; Stolińska-Fiedorowicz, H.; Kłys, W. Witaminy. In Normy dla populacji Polski; Jarosz, M., Ed.; IŻŻ: Warsaw, Poland, 2017; pp. 166–170. Available online: https://ncez.pl/upload/normy-net-1.pdf (accessed on 1 December 2020).
- Institute of Medicine. Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline; National Academies Press: Washington, DC, USA, 1998. [Google Scholar] [CrossRef]
- EFSA NDA Panel (EFSA Panel on Dietetic Products, Nutrition and Allergies). Scientific Opinion on Dietary Reference Values for folate. EFSA J. 2014, 12, 3893. [Google Scholar] [CrossRef]
- Bureau, S.; Mouhoubi, S.; Touloumet, L.; Garcia, C.; Moreau, F.; Bédouet, V.; Renard, C.M. Are folates, carotenoids and vitamin C affected by cooking? Four domestic procedures are compared on a large diversity of frozen vegetables. LWT 2015, 64, 735–741. [Google Scholar] [CrossRef]
- Pan, Z.; Sun, Y.; Zhang, F.; Guo, X.; Liao, Z. Effect of Thermal Processing on Carotenoids and Folate Changes in Six Varieties of Sweet Potato (Ipomoes batata L.). Foods 2019, 8, 215. [Google Scholar] [CrossRef] [Green Version]
- Petersen, M.A. Influence of sous vide processing, steaming and boiling on vitamin retention and sensory quality in broccoli florets. Eur. Food Res. Technol. 1993, 197, 375–380. [Google Scholar] [CrossRef] [PubMed]
- Seyoum, E.; Selhub, J. Properties of Food Folates Determined by Stability and Susceptibility to Intestinal Pteroylpolyglutamate Hydrolase Action. J. Nutr. 1998, 128, 1956–1960. [Google Scholar] [CrossRef]
- Hernandes, J.M.; Beardswort, P.M.; Weber, G. Egg quality–meeting consumer expectations. Int. J. Poult. 2005, 13, 20–23. Available online: http://www.positiveaction.info/pdfs/articles/pp13.3p20.pdf (accessed on 1 December 2020).
- Karadas, F.; Wood, N.A.; Surai, P.F.; Sparks, N.H. Tissue-specific distribution of carotenoids and vitamin E in tissues of newly hatched chicks from various avian species. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2005, 140, 506–511. [Google Scholar] [CrossRef]
- Grashorn, M.A.; Grimrath, J.Y.M. Unterscheidet sich die Qual-ität von Bio- und Käfigeiern? DGS Mag. 2005, 56, 18–25. [Google Scholar]
- Chowdhury, S.D.; Hassin, B.M.; Das, S.C.; Rashid, H.; Ferdaus, A.J. Evaluation of Marigold Flower and Orange Skin as Sources of Xanthophyll Pigment for the Improvement of Egg Yolk Color. J. Poult. Sci. 2008, 45, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Beardswort, P.M.; Hernandes, J.M. Yolk colour–an important egg quality attribute. Int. J. Poult. 2004, 12, 17–18. [Google Scholar]
- Patring, J.; Wandel, M.; Jägerstad, M.; Frølich, W. Folate content of Norwegian and Swedish flours and bread analysed by use of liquid chromatography–mass spectrometry. J. Food Compos. Anal. 2009, 22, 649–656. [Google Scholar] [CrossRef]
- Vahteristo, L.; Ollilainen, V.; Varo, P. HPLC Determination of Folate in Liver and Liver Products. J. Food Sci. 1996, 61, 524–526. [Google Scholar] [CrossRef]
- Konings, E.J.M. A Validated Liquid Chromatographic Method for Determining Folates in Vegetables, Milk Powder, Liver, and Flour. J. AOAC Int. 1999, 82, 119–127. [Google Scholar] [CrossRef] [Green Version]
- Pfeiffer, C.M.; Rogers, L.M.; Gregory, J.F. Determination of Folate in Cereal-Grain Food Products Using Trienzyme Extraction and Combined Affinity and Reversed-Phase Liquid Chromatography. J. Agric. Food Chem. 1997, 45, 407–413. [Google Scholar] [CrossRef]
- Jastrebova, J.; Witthöft, C.; Grahn, A.; Svensson, U.; Jägerstad, M. HPLC determination of folates in raw and processed beetroots. Food Chem. 2003, 80, 579–588. [Google Scholar] [CrossRef]
- Hefni, M.; Öhrvik, V.; Tabekha, M.; Witthöft, C. Folate content in foods commonly consumed in Egypt. Food Chem. 2010, 121, 540–545. [Google Scholar] [CrossRef]
- Blakley, R.L. The biochemistry of folic acid and related pteridines. In North-Holland Research Monographs; North-Holland Publishing Company: Amsterdam, The Netherlands, 1969; pp. 1–570. [Google Scholar]
- López, A.; Piqué, M.; Boatella, J.; Parcerisa, J.; Romero, A.; Ferrá, A.; Garcí, J. Influence of Drying Conditions on the Hazelnut Quality. III. Browning. Dry. Technol. 1997, 15, 989–1002. [Google Scholar] [CrossRef]



| Farming System | 5-CH3-H4Folate | 10-HCO-Folic Acid | Total Folates (Sum as Folic Acid) | Mean Total Folate Content as Folic Acid | |
|---|---|---|---|---|---|
| Organic | 1 1 | 101.4 ± 4.7 2 | 7.6 ± 0.3 | 104.9 ± 4.6 | 113.8 a3 |
| 2 | 91.8 ± 0.5 | 5.5 ± 0.1 | 93.7 ± 0.5 | ||
| 3 | 143.0 ± 1.1 | 5.1 ± 0.4 | 142.8 ± 1.3 | ||
| Free range | 1 | 88.0 ± 2.5 | 9.5 ± 0.5 | 93.8 ± 2.1 | 85.5 b |
| 2 | 92.3 ± 4.9 | 8.2 ± 0.2 | 96.7 ± 4.7 | ||
| 3 | 63.5 ± 2.8 | 5.1 ± 0.5 | 66.1 ± 2.5 | ||
| Barn | 1 | 86.4 ± 4.2 | 5.7 ± 0.2 | 88.6 ± 4.2 | 88.5 b |
| 2 | 92.3 ± 7.8 | 3.5 ± 0.3 | 92.5 ± 8.8 | ||
| 3 | 82.9 ± 1.0 | 4.7 ± 0.4 | 84.4 ± 1.1 | ||
| Caged | 1 | 78.4 ± 2.1 | 3.0 ± 0.2 | 78.4 ± 1.9 | 78.5 b |
| 2 | 75.2 ± 1.6 | 4.6 ± 0.2 | 76.9 ± 1.4 | ||
| 3 | 79.1 ± 2.3 | 4.2 ±.0.1 | 80.3 ± 3.2 | ||
| Group, Gender, Age, Years | ||||||
|---|---|---|---|---|---|---|
| Children 1–9 | Teenage Boys 10–18 | Teenage Girls 10–18 | Men and Women ≥ 19 | Pregnant and Breast-Feeding Women | ||
| µg Folate Equivalent/Person/Day | ||||||
| Farming System | RDA | 150–300 | 250–330 | 300–400 | 400 | 600 |
| Organic | Mean (µg/60g) | 68.3 | ||||
| DDC % | 23–46 | 21–27 | 17–23 | 17 | 11 | |
| Free range | Mean (µg/60g) | 51.3 | ||||
| DDC % | 17–34 | 16–21 | 13–17 | 13 | 9 | |
| Barn | Mean (µg/60g) | 53.1 | ||||
| DDC % | 18–35 | 16–21 | 13–18 | 13 | 9 | |
| Caged | Mean (µg/60g) | 47.1 | ||||
| DDC % | 16–31 | 14–19 | 12–16 | 12 | 8 | |
| Farming System | 5-CH3-H4 Folate (%) | 10-HCO-Folic Acid (%) | Total Folates (Sum as Folic Acid) (%) | |
|---|---|---|---|---|
| Organic | 1 1 | 12.0 2 | 25.8 | 13.0 |
| 2 | 7.1 | 44.8 | 9.1 | |
| 3 | 13.3 | 44.7 | 14.3 | |
| Free Range | 1 | 18.4 | 39.3 | 20.4 |
| 2 | 22.6 | 43.4 | 24.2 | |
| 3 | 14.2 | 11.9 | 14.0 | |
| Barn | 1 | 4.4 | 47.3 | 7.0 |
| 2 | 11.2 | 34.6 | 12.0 | |
| 3 | 1.7 | 52.1 | 4.3 | |
| Caged | 1 | 8.2 | 30.4 | 9.0 |
| 2 | 2.5 | 41.5 | 4.7 | |
| 3 | 7.5 | 44.5 | 9.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Czarnowska-Kujawska, M.; Draszanowska, A.; Gujska, E.; Klepacka, J.; Kasińska, M. Folate Content and Yolk Color of Hen Eggs from Different Farming Systems. Molecules 2021, 26, 1034. https://doi.org/10.3390/molecules26041034
Czarnowska-Kujawska M, Draszanowska A, Gujska E, Klepacka J, Kasińska M. Folate Content and Yolk Color of Hen Eggs from Different Farming Systems. Molecules. 2021; 26(4):1034. https://doi.org/10.3390/molecules26041034
Chicago/Turabian StyleCzarnowska-Kujawska, Marta, Anna Draszanowska, Elżbieta Gujska, Joanna Klepacka, and Marta Kasińska. 2021. "Folate Content and Yolk Color of Hen Eggs from Different Farming Systems" Molecules 26, no. 4: 1034. https://doi.org/10.3390/molecules26041034
APA StyleCzarnowska-Kujawska, M., Draszanowska, A., Gujska, E., Klepacka, J., & Kasińska, M. (2021). Folate Content and Yolk Color of Hen Eggs from Different Farming Systems. Molecules, 26(4), 1034. https://doi.org/10.3390/molecules26041034