Calcium Sulfite Solids Activated by Iron for Enhancing As(III) Oxidation in Water
Abstract
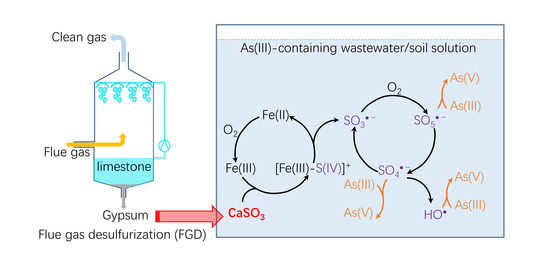
:1. Introduction
2. Results and Discussion
2.1. Control Experiments
2.2. Effects of Fe(III) and CaSO3 Dosages on As(III) Oxidation
2.3. Effect of pH on As(III) Oxidation
2.4. Effect of the Initial Concentration of As(III) on Its Oxidation
2.5. Contribution of Free Radicals to As(III) Oxidation
3. Materials and Methods
3.1. Materials
3.2. Reaction Procedure
3.3. Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Renew, J.E.; Huang, C.; Burns, S.E.; Carrasquillo, M.; Sun, W.; Ellison, K.M. Immobilization of heavy metals by solidification/stabilization of Co-disposed flue gas desulfurization brine and coal fly ash. Energy Fuels 2016, 30, 5042–5051. [Google Scholar] [CrossRef]
- Song, W.; Zhou, J.; Wang, B.; Li, S.; Cheng, R. Production of SO2 gas: New and efficient utilization of flue gas desulfurization gypsum and pyrite resources. Ind. Eng. Chem. Res. 2019, 58, 20450–20460. [Google Scholar] [CrossRef]
- Chi, C.; Zhao, C.; Sun, X.; Wang, Z. Reclamation of saline-sodic soil properties and improvement of rice (Oriza sativa L.) growth and yield using desulfurized gypsum in the west of Songnen Plain, northeast China. Geoderma 2012, 187, 24–30. [Google Scholar] [CrossRef]
- Lee, Y.B.; Bigham, J.M.; Dick, W.A.; Kim, P.J. Impact of flue gas desulfurization-calcium sulfite and gypsum on soil microbial activity and wheat growth. Soil Sci. 2008, 173, 534–543. [Google Scholar] [CrossRef]
- Nan, J.; Chen, X.; Chen, C.; Lashari, M.S.; Deng, J.; Du, Z. Impact of flue gas desulfurization gypsum and lignite humic acid application on soil organic matter and physical properties of a saline-sodic farmland soil in Eastern China. J. Soil Sediments 2016, 16, 2175–2185. [Google Scholar] [CrossRef]
- Yang, P.; Li, X.; Tong, Z.; Li, Q.; He, B.; Wang, L.; Guo, S.; Xu, Z. Use of flue gas desulfurization gypsum for leaching Cd and Pb in reclaimed tidal flat soil. Environ. Sci. Pollut. Res. 2016, 23, 7840–7848. [Google Scholar] [CrossRef]
- Schomberg, H.H.; Endale, D.M.; Jenkins, M.B.; Chaney, R.L.; Franklin, D.H. Metals in soil and runoff from a piedmont hay field amended with broiler litter and flue gas desulfurization gypsum. J. Environ. Qual. 2018, 47, 326–335. [Google Scholar] [CrossRef]
- Vaclavikova, M.; Gallios, G.; Hredzak, S.; Jakabsky, S. Removal of arsenic from water streams: An overview of available techniques. Clean Technol. Environ. Policy. 2008, 10, 89–95. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, N. Removal of carbamazepine from aqueous solution using sono-activated persulfate process. Ultrason. Sonochem. 2016, 29, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Su, R.; Wan, F.; Zhu, M.; Li, D.; Xu, S. Co3O4 nanocrystals on graphene oxide as a synergistic catalyst for degradation of Orange II in water by advanced oxidation technology based on sulfate radicals. Appl. Catal. B 2012, 123, 265–272. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Zhang, C.; Yu, Y.; Zhang, L.; Wu, F. A novel photochemical system of ferrous sulfite complex: Kinetics and mechanisms of rapid decolorization of Acid Orange 7 in aqueous solutions. Water Res. 2014, 57, 87–95. [Google Scholar] [CrossRef]
- Chen, L.; Peng, X.; Liu, J.; Li, J.; Wu, F. Decolorization of orange II in aqueous solution by an Fe(II)/sulfite system: Replacement of persulfate. Ind. Eng. Chem. Res. 2012, 51, 13632–13638. [Google Scholar] [CrossRef]
- Guo, Y.; Lou, X.; Fang, C.; Xiao, D.; Wang, Z.; Liu, J. Novel photo-sulfite system: Toward simultaneous transformations of inorganic and organic pollutants. Environ. Sci. Technol. 2013, 47, 11174–11181. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Xiao, M.; Zhang, L.; Wu, F.; Ge, L. Enhanced decolorization of orange II solutions by the Fe(II)–sulfite system under xenon lamp irradiation. Ind. Eng. Chem. Res. 2013, 52, 10089–10094. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, Y.; Yang, S.; Gao, H.; Chen, L. Roles of oxysulfur radicals in the oxidation of acid orange 7 in the Fe(III)–sulfite system. J. Sulfur Chem. 2015, 36, 373–384. [Google Scholar] [CrossRef]
- Zhou, D.; Chen, L.; Li, J.; Wu, F. Transition metal catalyzed sulfite auto-oxidation systems for oxidative decontamination in waters: A state-of-the-art minireview. Chem. Eng. Sci. 2018, 346, 726–738. [Google Scholar] [CrossRef]
- Bavasso, I.; Vilardi, G.; Stoller, M.; Chianese, A.; Di Palma, L. Perspectives in Nanotechnology Based Innovative Applications for The Environment. Chem. Eng. Trans. 2016, 47, 55–60. [Google Scholar]
- Di Palma, L.; Verdone, N.; Vilardi, G. Kinetic Modeling of Cr(VI) Reduction by nZVI in Soil: The Influence of Organic Matter and Manganese Oxide. Bull. Environ. Contam. Toxicol. 2018, 101, 692–697. [Google Scholar] [CrossRef]
- Xu, J.; Ding, W.; Wu, F.; Mailhot, G.; Zhou, D.; Hanna, K. Rapid catalytic oxidation of arsenite to arsenate in an iron(III)/sulfite system under visible light. Appl. Catal. B 2016, 186, 56–61. [Google Scholar] [CrossRef]
- Chen, L.; Tang, M.; Chen, C.; Chen, M.; Luo, K.; Xu, J.; Wu, F. Efficient bacterial inactivation by transition metal catalyzed auto-oxidation of sulfite. Environ. Sci. Technol. 2017, 51, 12663–12671. [Google Scholar] [CrossRef]
- Yuan, Y.; Luo, T.; Xu, J.; Li, J.; Wu, F. Enhanced oxidation of aniline using Fe(III)-S(IV) system: Role of different oxysulfur radicals. Chem. Eng. J. 2019, 362, 183–189. [Google Scholar] [CrossRef]
- Lee, Y.J.; Rochelle, G.T. Oxidative degradation of organic acid conjugated with sulfite oxidation in flue gas desulfurization: Products, kinetics, and mechanism. Environ. Sci. Technol. 1987, 21, 266–272. [Google Scholar] [CrossRef]
- Neta, P.; Robert, E.H.; Ross, A.B. Rate constants for reactions of inorganic radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1988, 17, 1027–1284. [Google Scholar] [CrossRef]
- Lente, G.; Fábián, I. Kinetics and mechanism of the oxidation of sulfur(IV) by iron(III) at metal ion excess. J. Chem. Soc. Dalton Trans. 2002, 5, 778–784. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, J.; Li, C.; Guo, S.; Wang, G. Reaction kinetics and mechanism of Iron(II)-induced catalytic oxidation of sulfur(IV) during wet desulfurization. Ind. Eng. Chem. Res. 2012, 51, 1158–1165. [Google Scholar] [CrossRef]
- Ge, J.; Zhou, Y.; Yang, Y.; Xue, M. Catalytic oxidative desulfurization of gasoline using Ionic liquid emulsion system. Ind. Eng. Chem. Res. 2012, 50, 13686–13692. [Google Scholar] [CrossRef]
- Shao, B.; Dong, H.; Sun, B.; Guan, X. Role of ferrate(IV) and ferrate(V) in activating ferrate(VI) by calcium sulfite for enhanced oxidation of organic contaminants. Environ. Sci. Technol. 2019, 53, 894–902. [Google Scholar] [CrossRef]
- Luo, T.; Peng, Y.; Chen, L.; Li, J.; Wu, F.; Zhou, D. Metal-Free Electro-activated sulfite process for As(III) oxidation in water using graphite electrodes. Env. Sci. Technol. 2020, 54, 10261–11026. [Google Scholar] [CrossRef]
- Xu, J.; Li, J.; Wu, F.; Zhang, Y. Rapid photooxidation of As(III) through surface complexation with nascent colloidal ferric hydroxide. Environ. Sci. Technol. 2014, 48, 272–278. [Google Scholar] [CrossRef]
- Nguyen, T.V.; Vigneswaran, S.; Ngo, H.H.; Kandasamy, J.; Choi, H.C. Arsenic removal by photo-catalysis hybrid system. Sep. Purif. Technol. 2008, 61, 44–50. [Google Scholar] [CrossRef]
- Patrick, M.; Jaromir, J.; Michèle, B. Degradation of diuron photoinduced by iron(III) in aqueous solution. Pesticide Sci. 1997, 49, 259–267. [Google Scholar]
- Qu, P.; Zhao, J.; Shen, T.; Hidaka, H. TiO2-assisted photodegradation of dyes: A study of two competitive primary processes in the degradation of RB in an aqueous TiO2 colloidal solution. J. Mol. Catal. A Chem. 1998, 129, 257–268. [Google Scholar] [CrossRef]
- Mclachlan, G.A.; Muller, J.G.; Rokita, S.E.; Burrows, C.J. Metal-mediated oxidation of guanines in DNA and RNA: A comparison of cobalt(II), nickel(II) and copper(II) complexes. Inorganica Chimica Acta. 1996, 251, 193–199. [Google Scholar] [CrossRef]
- Anipsitakis, G.P.; Dionysiou, D.D. Radical generation by the interaction of transition metals with common oxidants. Environ. Sci. Technol. 2004, 38, 3705–3712. [Google Scholar] [CrossRef]






| C0 | r0 | Kinetic Equation | kL−H | K | R2 |
|---|---|---|---|---|---|
| 1 | 0.1694 | 4.446 | 0.074 | 0.972 | |
| 3 | 0.6731 | ||||
| 5 | 1.3876 | ||||
| 8 | 1.7167 | ||||
| 10 | 1.7896 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, M.; Quan, S.; Li, J.; Wu, F.; Mailhot, G. Calcium Sulfite Solids Activated by Iron for Enhancing As(III) Oxidation in Water. Molecules 2021, 26, 1154. https://doi.org/10.3390/molecules26041154
Cai M, Quan S, Li J, Wu F, Mailhot G. Calcium Sulfite Solids Activated by Iron for Enhancing As(III) Oxidation in Water. Molecules. 2021; 26(4):1154. https://doi.org/10.3390/molecules26041154
Chicago/Turabian StyleCai, Minjuan, Sen Quan, Jinjun Li, Feng Wu, and Gilles Mailhot. 2021. "Calcium Sulfite Solids Activated by Iron for Enhancing As(III) Oxidation in Water" Molecules 26, no. 4: 1154. https://doi.org/10.3390/molecules26041154
APA StyleCai, M., Quan, S., Li, J., Wu, F., & Mailhot, G. (2021). Calcium Sulfite Solids Activated by Iron for Enhancing As(III) Oxidation in Water. Molecules, 26(4), 1154. https://doi.org/10.3390/molecules26041154