Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties
Abstract
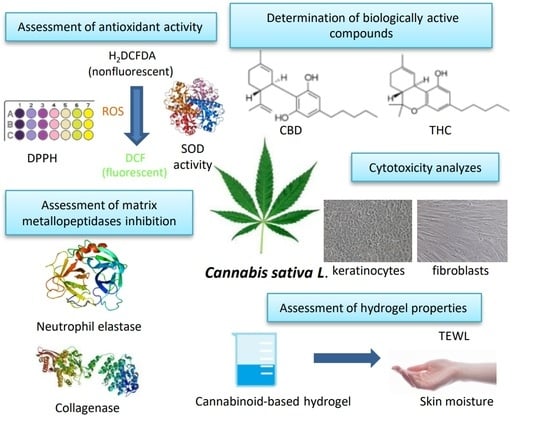
:1. Introduction
2. Results and Discussion
2.1. Determination of Biologically Active Compounds
2.2. Assessment of Antioxidant Activity
2.3. Cytotoxicity Assessment
2.4. Assessment of Matrix Metalloproteinases Inhibition
2.5. Assessment of Hydrogel Properties
3. Materials and Methods
3.1. Plant Material and Extraction Procedure
3.2. Determination of Biologically Active Compounds
3.2.1. Total Phenolic Content Determination
3.2.2. Total Flavonoids Content Determination
3.2.3. Determination of Chlorophyll Content
3.2.4. Determination of Cannabinoids
3.3. Assessment of Antioxidant Activity
3.3.1. DPPH Radical Scavenging Assay
3.3.2. Detection of Intracellular Levels of Reactive Oxygen Species (ROS)
3.3.3. Determination of Superoxide Dismutase (SOD) Activity
3.4. Cell Culture
3.5. Cell Viability Assay
3.5.1. Neutral Red Uptake Assay
3.5.2. Alamar Blue Assay
3.5.3. Lactate Dehydrogenase (LDH) Cytotoxicity Assay
3.6. Assessment of Matrix Metalloproteinases Inhibition
3.6.1. Determination of Anti-Collagenase Activity
3.6.2. Determination of Anti-Elastase Activity
3.7. Hydrogel Preparation
3.8. Transepidermal Water Loss (TEWL) and Skin Hydration Measurements
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Beg, S.; Swain, S.; Hasan, H.; Barkat, M.A.; Hussain, M.S. Systematic review of herbals as potential anti-inflammatory agents: Recent advances, current clinical status and future perspectives. Pharmacogn. Rev. 2011, 5, 120–137. [Google Scholar] [CrossRef] [Green Version]
- Cowan, M.M. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [Green Version]
- Mahlo, S.M.; Chauke, H.R.; McGaw, L.; Eloff, J. Antioxidant and antifungal activity of selected medicinal plant extracts against phytopathogenic fungi. Afr. J. Tradit. Complement. Altern. Med. 2016, 13, 216–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pisoschi, A.M.; Pop, A.; Cimpeanu, C.; Predoi, G. Antioxidant Capacity Determination in Plants and Plant-Derived Products: A Review. Oxid. Med. Cell Longev. 2016, 9130976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultana, B.; Anwar, F. Flavonols (kaempferol, quercetin, myricetin) contents of selected fruits, vegetables and medicinal plants. Food Chem. 2008, 108, 879–884. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Sridhar, C.; Reddy, Y.R.S.; De, B. Free radicals, antioxidants, diseases and phytomedicines: Current status and future prospect. Int. J. Pharm. Sci Rev. Res. 2010, 3, 91–100. [Google Scholar]
- Elser, P.; Maibach, H. Cosmeceuticals and Active Cosmetics; Taylor & Francis Group: New York, NY, USA, 2000. [Google Scholar]
- Nizioł-Łukaszewska, Z.; Furman-Toczek, D.; Zagórska–Dziok, M. Antioxidant activity and cytotoxicity of Jerusalem artichoke tubers and leaves extract on HaCaT and BJ fibroblast cells. Lipids Health Dis. 2018, 17, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cavalcanti, R.; Forster-Carneiro, T.; Gomes, M.; Rostagno, M.; Prado, J.; Meireles, M. Uses and applications of extracts from natural sources. In Natural Product Extraction: Principles and Applications; Royal Society of Chemistry: Cambridge, UK, 2013; pp. 1–3. [Google Scholar]
- Faccio, G. Plant Complexity and Cosmetic Innovation. iScience 2020, 23, 101358. [Google Scholar] [CrossRef] [PubMed]
- Bujak, T.; Wasilewski, T.; Nizioł-Łukaszewska, Z. Role of macromolecules in the safety of use of body wash cosmetics. Colloids Surf. B Biointerfaces 2015, 135, 497–503. [Google Scholar] [CrossRef]
- Barel, M.; Paye, M. Handbook of Cosmetic Science and Technology, 4th ed.; Taylor & Francis Group: Boca Raton, FL, USA, 2014; pp. 353–365. [Google Scholar]
- Rock, E.M.; Goodwin, J.M.; Limebeer, C.L.; Breuer, A.; Pertwee, R.G.; Mechoulam, R.; Parker, L.A. Interaction between non-psychotropic cannabinoids in marihuana: Effect of cannabigerol (CBG) on the anti-nausea or anti-emetic effects of cannabidiol (CBD) in rats and shrews. Psychopharmacology 2011, 215, 505–512. [Google Scholar] [CrossRef]
- Brenneisen, R. Chemistry and analysis of phytocannabinoids and other cannabis constituents. In Marijuana and the Cannabinoids; ElSohly, M.A., Ed.; Humana Press Inc.: Totowa, NJ, USA, 2007; pp. 17–50. [Google Scholar]
- Thomas, B.F.; Elsohly, M. Quality Assessment, Assurance, and Regulation of Medicinal Marijuana and Cannabinoid Preparations. Biosynthesis and Pharmacology of Phytocannabinoids and Related Chemical Constituents. In The Analytical Chemistry of Cannabis; Elsevier: Amsterdam, The Netherlands, 2016; Volume 2, pp. 27–41. [Google Scholar] [CrossRef]
- Russo, E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011, 163, 1344–1364. [Google Scholar] [CrossRef] [PubMed]
- Mańkowska, G.; Grabowska, L. Genetic resources of Cannabis sativa at the Institute of Natural Fibres and Medicinal Plants. Herba Pol. 2009, 55, 178–184. [Google Scholar]
- Borges, R.; Batista, J.; Viana, R.; Baetas, A.; Orestes, E.; Andrade, M.; da Silva, A. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elsohly, M.A.; Slade, D. Chemical constituents of marijuana: The complex mixture of natural cannabinoids. Life Sci. 2005, 78, 539–548. [Google Scholar] [CrossRef]
- Sharma, P.; Murthy, P.; Bharat, M.M.S. Chemistry, metabolism, and toxicology of Cannabis: Clinical implications. Iran. J. Psychiatry 2012, 7, 149–156. [Google Scholar]
- Whiting, P.F.; Wolff, R.F.; Deshpande, S.; Di Nisio, M.; Duffy, S.; Hernandez, A.V.; Keurentjes, J.C.; Lang, S.; Misso, K.; Ryder, S.; et al. Cannabinoids for medical use a systematic review and meta-analysis. JAMA 2015, 313, 2456–2473. [Google Scholar] [CrossRef]
- Grotenhermen, F. Pharmacology of cannabinoids. Neuro Endocrinol. Lett. 2004, 25, 14–23. [Google Scholar]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar] [CrossRef] [Green Version]
- Fukumoto, L.R.; Mazza, G. Assessing antioxidant and prooxidant activities of phenolic compounds. J. Agric. Food Chem. 2000, 48, 3597–3604. [Google Scholar] [CrossRef]
- Pollastro, F.; Minassi, A.; Fresu, L.G. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef]
- Koch, W.; Zagórska, J.; Marzec, Z.; Kukula-Koch, W. Applications of Tea (Camellia sinensis) and Its Active Constituents in Cosmetics. Molecules 2019, 24, 4277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izzo, L.; Castaldo, L.; Narváez, A.; Graziani, G.; Gaspari, A.; Rodríguez-Carrasco, Y.; Ritien, A. Analysis of Phenolic Compounds in Commercial Cannabis sativa L. Inflorescences Using UHPLC-Q-Orbitrap HRMS. Molecules 2020, 25, 631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maqsood, A.; Aatika, S.; Mazher, I.; Ansar, J.; Mingshan, I.; Qin, P.; Yuyang, L.; Zumin, G. Phytochemical screening, Total phenolics and Flavonoids Content and Antioxidant Activities of Citrullus colocynthis L. and Cannabis sativa L. Appl. Ecol. Environ. Res. 2019, 17, 6961–6979. [Google Scholar] [CrossRef]
- Dan, J.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inforescences, Leaves, Stem Barks, and Roots for Medicinal purposes. Sci. Rep. 2020, 24, 3309. [Google Scholar] [CrossRef]
- Mkpenie, V.; Essien, E.; Udoh, I. Effect of extraction conditions on total polyphenol contents, antioxidant and antimicrobial activities of Cannabis sativa L. Elec. J. Environ. Agric. Food Chem. 2012, 11, 300–307. [Google Scholar]
- Ferrante, C.; Recinella, L.; Maurizio, R.; Menghini, L.; Brunetti, L.; Chiavaroli, A.; Leone, S.; Iorio, L.; Carradori, S.; Tirillini, B.; et al. Multiple pharmacognostic characterization on hemp commercial cultivars: Focus on inflorescence water extract activity. Food Chem. Toxicol. 2019, 125, 452–461. [Google Scholar] [CrossRef]
- Agarwal, C.; Mathe, K.; Hofmann, T.; Csóka, L. Ultrasound-Assisted Extraction of Cannabinoids from Cannabis Sativa L. Optimized by Response Surface Methodology. J. Food Sci. 2018, 83, 700–710. [Google Scholar] [CrossRef]
- Hacke, A.C.M.; Lima, D.; de Costa, F.; Deshmukh, K.; Li, N.; Chow, A.M.; Marques, J.A.; Pereira, R.P.; Kerman, K. Probing the antioxidant activity of Δ9-tetrahydrocannabinol and cannabidiol in Cannabis sativa extracts. Analyst 2019, 144, 4952–4961. [Google Scholar] [CrossRef]
- Moldzio, R.; Pacher, T.; Krewenka, C.; Kranner, B.; Novak, J.; Duvigneau, J.C.; Rausch, W.D. Effects of cannabinoids Δ(9)-tetrahydrocannabinol, Δ(9)-tetrahydrocannabinolic acid and cannabidiol in MPP+ affected murine mesencephalic cultures. Phytomedicine 2012, 19, 819–824. [Google Scholar] [CrossRef]
- Allen, R.G. Oxidative stress and superoxide dismutase in development, aging and gene regulation. Age 1998, 21, 47–76. [Google Scholar] [CrossRef] [Green Version]
- Konca, Y.; Cimen, B.; Yalcin, H.; Kaliber, M.; Beyzi, S.B. Effect of Hemp Seed (Cannabis sativa sp.) Inclusion to the Diet on Performance, Carcass and Antioxidative Activity in Japanese Quail (Coturnix coturnix japonica). Korean J. Food Sci. Anim. Resour. 2014, 34, 141–150. [Google Scholar] [CrossRef] [Green Version]
- Coskun, Z.M.; Bolkent, S. Evaluation of Δ(9)-tetrahydrocannabinol metabolites and oxidative stress in type 2 diabetic rats. Iran. J. Basic Med. Sci. 2016, 19, 154–158. [Google Scholar] [PubMed]
- Repetto, G.; Sanz, P. Neutral red uptake, cellular growth and lysosomal function: In vitro effects of 24 metals. Altern. Lab. Anim. 1993, 21, 501–507. [Google Scholar] [CrossRef]
- Nemes, Z.; Dietz, R.; Luth, J.B. The pharmacological relevance of vital staining with neutral red. Experientia 1979, 35, 1475–1476. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, G.R.; Caton, M.C.; Nova, M.P.; Parandoosh, Z. Assessment of the Alamar Blue assay for cellular growth and viability in vitro. J. Immunol. Methods 1997, 204, 205–208. [Google Scholar] [CrossRef]
- O’Brien, J.; Wilson, I.; Orton, T.; Pognan, F. Investigation of the Alamar Blue (resazurin) fluorescent dye for the assessment of mammalian cell cytotoxicity. Eur. J. Biochem. 2000, 267, 5421–5426. [Google Scholar] [CrossRef]
- Park, M.V.; Lankveld, D.P.; van Loveren, H.; de Jong, W.H. The status of in vitro toxicity studies in the risk assessment of nanomaterials. Nanomedicine 2009, 4, 669–685. [Google Scholar] [CrossRef]
- Patravale, V.; Dandekar, P.; Jain, R. Nanotoxicology: Evaluating toxicity potential of drug-nanoparticles. Nanoparticulate Drug Delivery 2012, 4, 123–155. [Google Scholar] [CrossRef]
- Navarro, G.; Reyes-Resina, I.; Rivas-Santisteban, R.; de Medina, V.S.; Morales, P.; Cassano, S.; Ferreiro-Vera, C.; Lillo, A.; Aguinaga, D.; Jagerovic, N.; et al. Cannabidiol skews biased agonism at cannabinoid CB1 and CB2 receptors with smaller effect in CB1-CB2 heteroreceptor complexes. Biochem. Pharmacol. 2018, 157, 148–158. [Google Scholar] [CrossRef]
- Pastar, I.; Stojadinovic, O.; Yin, N.C.; Ramirez, H.; Nusbaum, A.G.; Sawaya, A.; Tomic-Canic, M. Epithelialization in wound healing: A comprehensive review. Adv. Wound Care 2014, 3, 445–464. [Google Scholar] [CrossRef] [Green Version]
- Marina, M.E.; Roman, I.I.; Constantin, A.M.; Mihu, C.M.; Tataru, A.D. VEGF involvement in psoriasis. Clujul Med. 2015, 88, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Hanel, K.H.; Cornelissen, C.; Luscher, B.; Baron, J.M. Cytokines and the skin barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef] [Green Version]
- Siurkus, J.; Peciura, R. The Topical Composition with Active Compounds from Cannabis sativa and Calendula Officinalis for Reduction of Skin Lesions. U.S. Patent WO2017175126, 12 October 2017. [Google Scholar]
- Bisogno, T.; Hanus, L.; De Petrocellis, L.; Tchilibon, S.; Ponde, D.E.; Brandi, I.; Moriello, A.S.; Davis, J.B.; Mechoulam, R.; Di Marzo, V. Molecular targets for cannabidiol and its synthetic analogues: Effect on vanilloid VR1 receptors and on the cellular uptake and enzymatic hydrolysis of anandamide. Br. J. Pharmacol. 2001, 134, 845–852. [Google Scholar] [CrossRef]
- Jarocka-Karpowicz, I.; Biernacki, M.; Wroński, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Effects on Phospholipid Metabolism in Keratinocytes from Patients with Psoriasis Vulgaris. Biomolecules 2020, 10, 367. [Google Scholar] [CrossRef] [Green Version]
- Sangiovanni, E.; Fumagalli, M.; Pacchetti, B.; Piazza, S.; Magnavacca, A.; Khalilpour, S.; Melzi, G.; Martinelli, G.; Dell’Agli, M. Cannabis sativa L. extract and cannabidiol inhibit in vitro mediators of skin inflammation and wound injury. Phytother. Res. 2019, 33, 2083–2093. [Google Scholar] [CrossRef]
- Petrosino, S.; Verde, R.; Vaia, M.; Allarà, M.; Iuvone, T.; Di Marzo, V. 2-Pentadecyl-2-Oxazoline, the Oxazoline of Pea, Modulates Carrageenan-Induced Acute Inflammation. Front. Pharmacol. 2017, 8, 308. [Google Scholar] [CrossRef] [Green Version]
- Song, R.; Bian, H.N.; Lai, W.; Chen, H.D.; Zhao, K.S. Normal skin and hypertrophic scar fibroblasts differentially regulate collagen and fibronectin expression as well as mitochondrial membrane potential in response to basic fibroblast growth factor. Braz. J. Med. Biol Res. 2011, 44, 402–410. [Google Scholar] [CrossRef]
- Rawal, S.Y.; Dabbous, M.K.; Tipton, D.A. Effect of cannabidiol on human gingival fibroblast extracellular matrix metabolism: MMP production and activity, and production of fibronectin and transforming growth factor β. J. Periodontal Res. 2012, 47, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Leyden, J.J.; Rewlings, A.V. Skin Moisturization. Cosmetics Science and Technology Series; Marcel Dekker: New York, NY, USA, 2002; pp. 223–245. [Google Scholar]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the Treatment of Chronic Pruritus: A Review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef] [PubMed]
- Sheriff, T.; Lin, M.J.; Dubin, D.; Khorasani, H. The potential role of cannabinoids in dermatology. J. Dermatol. Treat. 2019, 8, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Eagelston, L.R.M.; Yazd, N.K.K.; Patel, R.R.; Flaten, H.K.; Dunnick, C.A.; Dellavalle, R.P. Cannabinoids in dermatology: A scoping review. Dermatol. Online J. 2018, 24, 1–17. [Google Scholar]
- Oláh, A.; Tóth, B.I.; Borbíró, I. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Investig. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seweryn, A. Interactions between surfactants and the skin—Theory and practice. Adv. Coll. Int. Sci. 2018, 256, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Liu, X.; Gao, Y. Extraction optimization of bioactive compounds crocin, geniposide and total phenolic compounds from Gardenia Gardenia jasminoides Ellis fruits with response surface methodology. Innov. Food Sci. Emerg. Technol. 2009, 10, 610–615. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.J. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Matejić, J.S.; Džamić, A.M.; Mihajilov-Krstev, T.M.; Ranđelović, V.N.; Krivošej, Z.D.; Marin, P.D. Total phenolic and flavonoid content, antioxidant and antimicrobial activity of extracts from Tordylium maximum. J. Appl. Pharm. Sci. 2013, 3, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Borenfreund, E.; Puerner, J.A. Toxicity determined in vitro by morphological alterations and neutral red absorption. Toxicol. Lett. 1985, 24, 119–124. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A new fluorometric assay for cytotoxicity measurements in-vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]









| TPC [mg GAE/g DWE] | TFC [mg QE/g DWE] | Chlorophyll a [mg/g DWE] | Chlorophyll b [mg/g DWE] | Chlorophyll a + b [mg/g DWE] | |
|---|---|---|---|---|---|
| MAE | 42.524 ± 0.005 a | 8.091 ± 0.010 a | 1.923 ± 0.04 a | 0.241 ± 0.015 a | 2.642 ± 0.023 a |
| UAE | 51.322 ± 0.012 b | 10.374 ± 0.009 b | 4.372 ± 0.022 b | 0.821 ± 0.010 b | 5.404 ± 0.042 b |
| TPC [mg GAE/g DWH] | TFC [mg QE/g DWH] | Chlorophyll a [mg/g DWH] | Chlorophyll b [mg/g DWH] | Chlorophyll a + b [mg/g DWH] | |
| MAE | 2.511 ± 0.011 a | 0.483 ± 0.011 a | 0.113 ± 0.005 a | 0.014 ± 0.016 a | 0.156 ± 0.019 a |
| UAE | 3.184 ± 0.008 b | 0.642 ± 0.005 b | 0.271 ± 0.009 b | 0.051 ± 0.015 b | 0.335 ± 0.022 b |
| Chemical Compound | MAE [mg/g DW] | UAE [mg/g DW] |
|---|---|---|
| Cannabidiol (CBD) | 12.00 ± 1.43 | 31.00 ± 2.86 |
| Cannabidiol acid (CBD-A) | 130.00 ± 1.92 | 150.00 ± 16.84 |
| Cannabigerol (CBG) | Not detected | Not detected |
| Cannabigerolic acid (CBG-A) | 4.20 ± 0.38 | 6.30 ± 0.52 |
| Delta-9-tetrahydrocannabinol (THC) | 2.60 ± 0.19 | 4.00 ± 0.34 |
| Tetrahydrocannabinolic acid (THC-A) | 4.10 ± 0.46 | 6.50 ± 0.52 |
| Cannabinol (CBN) | Not detected | Not detected |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zagórska-Dziok, M.; Bujak, T.; Ziemlewska, A.; Nizioł-Łukaszewska, Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules 2021, 26, 802. https://doi.org/10.3390/molecules26040802
Zagórska-Dziok M, Bujak T, Ziemlewska A, Nizioł-Łukaszewska Z. Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules. 2021; 26(4):802. https://doi.org/10.3390/molecules26040802
Chicago/Turabian StyleZagórska-Dziok, Martyna, Tomasz Bujak, Aleksandra Ziemlewska, and Zofia Nizioł-Łukaszewska. 2021. "Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties" Molecules 26, no. 4: 802. https://doi.org/10.3390/molecules26040802
APA StyleZagórska-Dziok, M., Bujak, T., Ziemlewska, A., & Nizioł-Łukaszewska, Z. (2021). Positive Effect of Cannabis sativa L. Herb Extracts on Skin Cells and Assessment of Cannabinoid-Based Hydrogels Properties. Molecules, 26(4), 802. https://doi.org/10.3390/molecules26040802