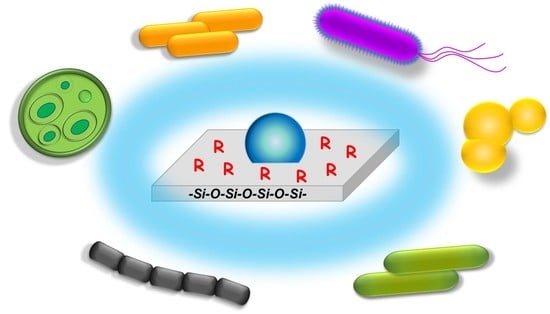
New Antiadhesive Hydrophobic Polysiloxanes
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Properties of the Functionalized Polysiloxanes
2.2. Antiadhesive Properties of the Functionalized Silicones
3. Materials and Methods
3.1. Synthesis of Functionalized Polysiloxanes
3.1.1. Synthesis of poly[2-(carboxymethylthioethyl)methylsiloxane] (P-1)
3.1.2. A General Procedure for the Synthesis of poly[2-(mercaptoethylamidomethylthioethyl)methylsiloxane] (P-2) and poly[2-(n-propylamidomethylthioethyl)methylsiloxane] (P-3)
P-2
P-3
3.2. Analytic Methods
3.2.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
3.2.2. Fourier Transform Infra-Red Spectroscopy (FT-IR)
3.2.3. Thermogravimetric Analysis (TGA)
3.2.4. Differential Scanning Calorimetry (DSC)
3.2.5. Surface Energy Measurements
3.3. Biological Studies
3.3.1. Biological Material
3.3.2. Abiotic Surfaces
3.3.3. Assessment of Bacterial Adhesion
3.3.4. Determination of Antimicrobial Activity of Working Solutions Used to Create Functional Polymers and Other Model Compounds
3.3.5. Statistical Methods
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jones, R.G.; Ando, W.; Chojnowski, J. (Eds.) Silicon-Containing Polymers. The Science and Technology of Their Synthesis and Applications; Springer Science+Business Media: Berlin, Germany, 2000; Section 1; pp. 1–244. [Google Scholar] [CrossRef]
- Abe, Y.; Gunji, T. Oligo- and polysiloxanes. Progr. Polym. Sci. 2004, 29, 149–182. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Curtis, J.; Colas, A. Chapter II. 5.18—Medical applications of silicones A2—Ratner, Buddy, D. In Biomaterials Science, 3rd ed.; Hoffman, A.S., Schoen, F.J., Lemons, J.E., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 1106–1116. ISBN 9780080877808. [Google Scholar]
- Mojsiewicz-Pieńkowska, K. Safety and Toxicity Aspects of Polysiloxanes (Silicones) Applications. Chapter 16 in Concise Encylopedia of High Performance Silicones; Tiwari, A., Soucek, M.D., Eds.; Scrivener Publishing LLC.: Beverly, MA, USA, 2014; pp. 243–252. ISBN 9781118938478. [Google Scholar] [CrossRef]
- Mazurek, M.H. 3.12 Silicones. In Comprehensive Organometallic Chemistry III; Michael, D., Mingos, P., Crabtree, R.H., Eds.; Elsevier: Oxford, UK, 2007; pp. 651–697. ISBN 978-0-08-045047-6. [Google Scholar]
- Owen, M.J. Silicone hydrophobicity and oleophilicity. Silicon 2014, 9, 651–655. [Google Scholar] [CrossRef]
- Krishnan, S.; Weinman, C.J.; Ober, C.K. Advances in polymers for anti-biofouling surfaces. J. Mater. Chem. 2008, 18, 3405–3413. [Google Scholar] [CrossRef]
- Yuan, Y.; Hays, M.P.; Hardwidge, P.R.; Kim, J. Surface characteristics influencing bacterial adhesion to polymeric substrates. RSC Adv. 2017, 7, 14254–14261. [Google Scholar] [CrossRef] [Green Version]
- Lam, M.; Migonney, V.; Falentin-Daudre, C. Review of silicone surface modification techniques and coatings for antibacterial/antimicrobial applications to improve breast implant surfaces. Acta Biomater. 2021, 119, 42–56. [Google Scholar] [CrossRef]
- Chen, A.; Peng, H.; Blakey, I.; Whittaker, A.K. Biocidal Polymers: A Mechanistic Overview. Polym. Rev. 2007, 57, 276–310. [Google Scholar] [CrossRef]
- Muñoz-Bonilla, A.; Fernandez-Garcia, M. Polymeric materials with antimicrobial activity. Progr. Polym. Sci. 2012, 37, 281–339. [Google Scholar] [CrossRef]
- Kottmann, A.; Mejίa, E.; Hémery, T.; Klein, J.; Kragl, U. Recent Developments in the Preparation of Silicones with Antimicrobial Properties. Chem. Asian J. 2017, 12, 1168–1179. [Google Scholar] [CrossRef]
- Ganewatta, M.S.; Chuanbing Tang, C. Controlling macromolecular structures towards effective antimicrobial polymers. Polymer 2015, 63, A1–A29. [Google Scholar] [CrossRef]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Progr. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.L.; Faÿ, F.; Réhel, K.; Linossier, I.; Grunlan, M.A. Bacteria and diatom resistance of silicones modified with PEO-silane amphiphiles. Biofouling 2014, 30, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, M.L.; Schott, S.S.; Grigoryan, B.; Rufin, M.A.; Ngo, B.K.D.; Vanderwal, L.; Stafslien, S.J.; Grunlan, M.A. Anti-protein and anti-bacterial behavior of amphiphilic silicones. Polym. Chem. 2017, 8, 5239–5251. [Google Scholar] [CrossRef]
- Kuliasha, C.A.; Finlay, J.A.; Franco, S.C.; Clare, A.S.; Stafslien, S.J.; Brennan, A.B. Marine anti-biofouling efficacy of amphiphilic poly(coacrylate) grafted PDMSe: Effect of graft molecular weight. Biofouling 2017, 33, 252–267. [Google Scholar] [CrossRef]
- Guazzelli, E.; Galli, G.; Martinelli, E.; Margaillan, A.; Bressy, C. Amphiphilic hydrolyzable polydimethylsiloxane-b-poly(ethyleneglycol methacrylate-co-trialkylsilyl methacrylate) block copolymers for marine coatings. I. Synthesis, hydrolysis and surface wettability. Polymer 2020, 186, 121954. [Google Scholar] [CrossRef]
- Fang, K.; Park, O.-J.; Hong, S.H. Controlling biofilms using synthetic biology approaches. Biotechnol. Adv. 2020, 40, 107518. [Google Scholar] [CrossRef]
- Kim, Y.D.; Dordick, J.S.; Clark, D.S. Siloxane-Based Biocatalytic Films and Paints for Use as Reactive Coatings. Biotechnol. Bioeng. 2001, 72, 475–482. [Google Scholar] [CrossRef]
- Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. Poly(silsesquioxanes) and poly(siloxanes) grafted with N-acetylcysteine for eradicating mature bacterial biofilms in water environment. Colloids Surf. B Biointerfaces 2018, 172, 627–634. [Google Scholar] [CrossRef] [PubMed]
- Kregiel, D.; Rygala, A.; Kolesinska, B.; Nowacka, M.; Herc, A.S.; Kowalewska, A. Antimicrobial and antibiofilm n-acetyl-l-cysteine grafted siloxane polymers with potential for use in water systems. Int. J. Mol. Sci. 2019, 20, 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drozdov, F.V.; Tarasenkov, A.N.; Parshina, M.S.; Cherkaev, G.V.; Strukova, E.N.; Muzafarov, A.M. Synthesis of guanidinopropyl triethoxysilane and its homopolymer as a new class of organosilicon antibacterial agents. J. Organomet. Chem. 2020, 918, 121243. [Google Scholar] [CrossRef]
- Obad, J.; Šušković, J.; Kos, B. Antimicrobial activity of ibuprofen: New perspectives on an “Old” non-antibiotic drug. Eur. J. Pharm. Sci. 2015, 71, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Fraise, A.P.; Wilkinson, M.A.C.; Bradley, C.R.; Oppenheim, B.; Moiemen, N. The antibacterial activity and stability of acetic acid. J. Hosp. Infect. 2013, 84, 329–331. [Google Scholar] [CrossRef]
- Halstead, F.D.; Rauf, M.; Moiemen, N.S.; Bamford, A.; Wearn, C.M.; Fraise, A.P.; Lund, P.A.; Oppenheim, B.A.; Webber, M.A. The Antibacterial Activity of Acetic Acid against Biofilm-Producing Pathogens of Relevance to Burns Patients. PLoS ONE 2015, 10, e0136190. [Google Scholar] [CrossRef] [Green Version]
- Jebors, S.; Pinese, C.; Nottelet, B.; Parra, K.; Amblard, M.; Mehdi, A.; Martinez, J.; Subra, G. Turning peptides in comb silicone polymers. J. Pept. Sci. 2015, 21, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.; Wehbi, M.; Echalier, C.; Hunger, S.; Bethry, A.; Garric, X.; Pinese, C.; Martinez, J.; Vezenkov, L.; Subra, G.; et al. Direct Synthesis of Peptide-Containing Silicones: A New Way to Bioactive Materials. Chem. Eur. J. 2020, 26, 12839–12845. [Google Scholar] [CrossRef]
- Trivedi, M.V.; Laurence, J.S.; Siahaan, T.J. The role of thiols and disulfides in protein chemical and physical stability. Curr. Protein Pept. Sci. 2009, 10, 614–625. [Google Scholar] [CrossRef] [Green Version]
- Costa, F.; Sousa, D.M.; Parreira, P.; Lamghari, M.; Gomes, P.; Martins, M.C.L. N-acetylcysteine-functionalized coating avoids bacterial adhesion and biofilm formation. Sci. Rep. 2017, 7, 17374. [Google Scholar] [CrossRef] [Green Version]
- Kowalewska, A.; Nowacka, M.; Tracz, A.; Makowski, T. Supramolecular self-assembly of linear oligosilsesquioxanes on mica-AFM surface imaging and hydrophilicity studies. Soft Matter 2015, 11, 4818–4829. [Google Scholar] [CrossRef]
- Comerford, J.W.; Clark, J.H.; Macquarrie, D.J.; Breeden, S.W. Clean, reusable and low cost heterogeneous catalyst for amide synthesis. Chem. Commun. 2009, 2562–2564. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Makowski, T.; Kowalewska, A. Hybrid Fluorescent Poly(silsesquioxanes) with Amide- and Triazole-Containing Side Groups for Light Harvesting and Cation Sensing. Materials 2020, 13, 4491. [Google Scholar] [CrossRef]
- Dünki, S.J.; Cuervo-Reyes, E.; Opris, D.M. A facile synthetic strategy to polysiloxanes containing sulfonyl side groups with high dielectric permittivity. Polym. Chem. 2017, 8, 715–724. [Google Scholar] [CrossRef] [Green Version]
- Thomas, T.H.; Kendrick, T.C. Thermal analysis of polydimethylsiloxanes. I. Thermal degradation in controlled atmospheres. J. Polym. Sci. Part A2 Polym. Phys. 1969, 7, 537–549. [Google Scholar] [CrossRef]
- Camino, G.; Lomakin, S.M.; Lageard, M. Thermal polydimethylsiloxane degradation. Part 2. The degradation mechanisms. Polymer 2002, 43, 2011–2015. [Google Scholar] [CrossRef]
- Fina, A.; Tabuani, D.; Carniato, F.; Frache, A.; Boccaleri, E.; Camino, G. Polyhedral oligomeric silsesquioxanes (POSS) thermal degradation. Thermochim. Acta 2006, 440, 36–42. [Google Scholar] [CrossRef]
- Nowacka, M.; Fischer, C.; Kowalewska, A.; Hebda, M.; Hodor, K. Thermally induced phenomena leading to degradation of poly(silsesquioxane) materials. Eur. Polym. J. 2017, 86, 17–28. [Google Scholar] [CrossRef]
- Kowalewska, A.; Nowacka, M.; Makowski, T.; Michalski, A. Thermal stability of self-assembled surfaces and micropatterns made of ladder polysilsesquioxanes. Polymer 2016, 90, 147–155. [Google Scholar] [CrossRef]
- Männle, F.; Rosquist Tofteberg, T.; Skaugen, M.; Bu, H.; Peters, T.; Dietzel, P.D.C.; Pilz, M. Polymer nanocomposite coatings based on polyhedral oligosilsesquioxanes: Route for industrial manufacturing and barrier properties. J. Nanopart. Res. 2011, 13, 4691–4701. [Google Scholar] [CrossRef]
- Søndergaard, R.R.; Norrman, K.; Krebs, F.C. Low-temperature side-chain cleavage and decarboxylation of polythiophene esters by acid catalysis. J. Polym. Sci. Part A Polym. Chem. 2012, 50, 1127–1132. [Google Scholar] [CrossRef]
- Wu, W.H.; Thomas, P.; Hume, P.; Jin, J. Effective Conversion of Amide to Carboxylic Acid on Polymers of Intrinsic Microporosity (PIM-1) with Nitrous Acid. Membranes 2018, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Voronkov, M.G. Reactions of α-elimination of silanones as a path for formation and destruction of siloxane structures. Russ. Chem. Bull. 1998, 47, 795–806. [Google Scholar] [CrossRef]
- Dong, F.; Tang, X.; Ma, L.; Tan, X.; Feng, S. Thermal degradation kinetics of functional polysiloxane with pendent γ-chloropropyl groups. Polym. Bull. 2020, in press. [Google Scholar] [CrossRef]
- Zhang, K.; Ishida, H. Smart Synthesis of High-Performance Thermosets Based on ortho-Amide–Imide Functional Benzoxazines. Front. Mater. 2015, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Kotliar, A.M.J. Interchange reactions involving condensation polymers. Polym. Sci. Macromol. Rev. 1981, 16, 367–395. [Google Scholar] [CrossRef]
- Van Bennekom, A.C.M.; Willemsen, P.A.A.T.; Gaymans, R.J. Amide-modified poly(butylene terepthalate): Thermal stability. Polymer 1996, 37, 5447–5459. [Google Scholar] [CrossRef] [Green Version]
- Nowacka, M.; Herc, A.S.; Kowalewska, A. Thiol-ene addition of mercaptoalcohols to poly(vinylsiloxanes) under visible light photocatalysis—An approach towards cross-linkable hydrophilic silicones. Polyhedron 2020, 185, 114588. [Google Scholar] [CrossRef]
- Kowalewska, A.; Nowacka, M. Synthesis of Ladder Silsesquioxanes by in situ Polycondensation of Cyclic Tetravinylsiloxanetetraols. Silicon 2015, 7, 133–146. [Google Scholar] [CrossRef] [Green Version]
- Kręgiel, D.; Berlowska, J.; Mizerska, U.; Fortuniak, W.; Chojnowski, J.; Ambroziak, W. Chemical modification of polyvinyl chloride and silicone elastomer in inhibiting adhesion of Aeromonas hydrophila. World J. Microbiol. Biotechnol. 2013, 29, 1197–1206. [Google Scholar] [CrossRef] [Green Version]
- Zi, Y.; Zhu, M.; Li, X.; Xu, Y.; Wei, H.; Li, D.; Mu, C. Effects of carboxyl and aldehyde groups on the antibacterial activity of oxidized amylose. Carbohydr. Polym. 2018, 192, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Köllnberger, A.; Schrader, R.; Briehn, C.A. Carboxylic acid mediated antimicrobial activity of silicone elastomers. Mater. Sci. Eng. C 2020, 113, 111001. [Google Scholar] [CrossRef]
- Peterson, L.R. Quinolone molecular structure-activity relationships: What we have learned about improving antimicrobial activity. Clin. Infect. Dis. 2001, 33 (Suppl. 3), S180–S186. [Google Scholar] [CrossRef]
- Wiradharma, N.; Khan, M.; Yong, L.-K.; Hauser, C.A.E.; Seow, S.V.; Zhang, S.; Yang, Y.-Y. The effect of thiol functional group incorporation into cationic helical peptides on antimicrobial activities and spectra. Biomaterials 2011, 32, 9100–9108. [Google Scholar] [CrossRef] [PubMed]
- Matthysse, A.G. Exopolysaccharides of Agrobacterium tumefaciens. In Agrobacterium Biology; Springer: Berlin/Heidelberg, Germany, 2018; pp. 111–141. [Google Scholar]
- Sabev, H.A.; Robson, G.D.; Handley, P.S. Influence of starvation, surface attachment and biofilm growth on the biocide susceptibility of the biodeteriogenic yeast Aureobasidium pullulans. J. Appl. Microbiol. 2006, 101, 319–330. [Google Scholar] [CrossRef]
- Dos Santos, V.L.; Monteiro Ade, S.; Braga, D.T.; Santoro, M.M. Phenol degradation by Aureobasidium pullulans FE13 isolated from industrial effluents. J. Hazard. Mater. 2009, 161, 1413–1420. [Google Scholar] [CrossRef] [PubMed]
- Leathers, T.D.; Rich, J.O.; Nunnally, M.S.; Anderson, A.M. Inactivation of virginiamycin by Aureobasidium pullulans. Biotechnol. Lett. 2018, 40, 157–163. [Google Scholar] [CrossRef]
- Sevilla, M.J.; Landajuela, L.; Uruburu, F. The effect of alcohols on the morphology of Aureobasidium pullulans. Curr. Microbiol. 1983, 9, 169–171. [Google Scholar] [CrossRef]
- Campbell, B.S.; Siddique, A.B.M.; McDougall, B.M.; Seviour, R.J. Which morphological forms of the fungus Aureobasidium pullulans are responsible for pullulan production? FEMS Microbiol. Lett. 2004, 232, 225–228. [Google Scholar] [CrossRef] [Green Version]
- Armarego, W.L.F.; Chai, C.L.L. Purification of Laboratory Chemicals, 5th ed.; Elsevier Science: London, UK, 2003. [Google Scholar]
- Kowalewska, A.; Fortuniak, W.; Rózga-Wijas, K.; Handke, B. Thermolysis of new hybrid silsesquioxane-carbosilane materials. Thermochim. Acta 2009, 494, 45–53. [Google Scholar] [CrossRef]
- Owens, D.K.; Wendt, R.C. Estimation of the surface free energy of polymers. J. Appl. Polym. Sci. 1969, 13, 1741–1747. [Google Scholar] [CrossRef]
- Rygala, A.; Berlowska, D.; Kregiel, D. Heterotrophic plate count for bottled water safety management. Processes 2020, 8, 739. [Google Scholar] [CrossRef]
- Wong, Y.K.; Ho, Y.H.; Ho, K.C.; Leung, H.M.; Yung, K.K.L. Growth medium screening for Chlorella vulgaris growth and lipid production. J. Aquac. Mar. Biol. 2017, 6, 00143. [Google Scholar] [CrossRef] [Green Version]
- Kregiel, D. Attachment of Asaia lannensis to materials commonly used in beverage industry. Food Control 2013, 32, 537–542. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| Sample | T5% (°C) | Td1 (°C) | Td2 (°C) | Td3 (°C) | Rate (%∙min/°C) | Residue (%) | ||
|---|---|---|---|---|---|---|---|---|
| Vd1 | Vd2 | Vd3 | ||||||
| VMS-T11 | 87.6 | 117.2 | 272.0 | 554.1 | 8.41 | 4.59 | 0.38 | 10.1 |
| P-1 | 137.6 | 135.6 | 350.3 | 506.5 | 0.30 | 5.77 | 0.34 | 33.4 |
| P-2 | 135.4 | 134.1 | 238.2 | 357.4 | 0.81 | 6.10 | 2.77 | 32.7 |
| P-3 | 129.7 | 159.5 | 256.7 | 361.9 | 1.73 | 0.71 | 5.80 | 33.3 |
| Strain | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| A. hydrophila | 0 | 0 | 0 | 35 | 38 | 40 | 18 | 16 |
| A. tumefaciens | 0 | 0 | 0 | 30 | 33 | 30 | 12 | 14 |
| E. coli | 0 | 0 | 0 | 10 | 23 | 29 | 11 | 10 |
| S. aureus | 0 | 0 | 0 | 28 | 27 | 27 | 8 | 10 |
| A. pullulans | 0 | 0 | 0 | 33 | 32 | 36 | 7 | 15 |
| C. vulgaris | 0 | 0 | 0 | 28 | 17 | 13 | 8 | 10 |
| Sample | P-2 | P-3 |
|---|---|---|
| P-1 (g) | 3 | 3 |
| cysteamine (mol) | 0.0168 | - |
| n-propylamine (mol) | - | 0.0168 |
| [COOH]0/[NH2]0 THF (mL) toluene (mL) DMF (mL) SiO2 (g) X (%) Y (%) | 1 10 90 45 0.4 72 56 | 1 10 80 - 0.4 63 52 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowacka, M.; Rygała, A.; Kręgiel, D.; Kowalewska, A. New Antiadhesive Hydrophobic Polysiloxanes. Molecules 2021, 26, 814. https://doi.org/10.3390/molecules26040814
Nowacka M, Rygała A, Kręgiel D, Kowalewska A. New Antiadhesive Hydrophobic Polysiloxanes. Molecules. 2021; 26(4):814. https://doi.org/10.3390/molecules26040814
Chicago/Turabian StyleNowacka, Maria, Anna Rygała, Dorota Kręgiel, and Anna Kowalewska. 2021. "New Antiadhesive Hydrophobic Polysiloxanes" Molecules 26, no. 4: 814. https://doi.org/10.3390/molecules26040814
APA StyleNowacka, M., Rygała, A., Kręgiel, D., & Kowalewska, A. (2021). New Antiadhesive Hydrophobic Polysiloxanes. Molecules, 26(4), 814. https://doi.org/10.3390/molecules26040814