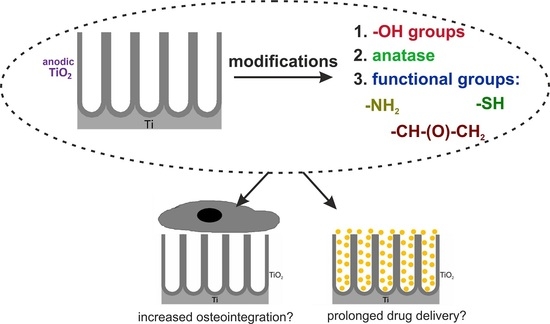
The Impacts of Crystalline Structure and Different Surface Functional Groups on Drug Release and the Osseointegration Process of Nanostructured TiO2
Abstract
:1. Introduction
2. Results and Discussion
2.1. The Effect of the Crystalline Structure on the ATO Modification with NaOH
2.2. Surface Modifications of Annealed TiO2 Layers with Silane Derivatives
2.3. Effects of the Surface Modification on Ibuprofen Release Kinetics
2.4. Biocompatibility of the Modified ATO Layers
3. Materials and Methods
3.1. Synthesis of Nanostructured Titanium Dioxide on Ti Substrate
3.2. Modification of Surface and Crystalline Structure of TiO2 Layers
3.3. Ibuprofen Delivery Process
3.4. Cell Cultivation on Studied ATO Layers
3.5. Examination of the Metabolic Activity and Morphology of MG-63 Cells
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Awad, N.K.; Edwards, S.L.; Morsi, Y.S. A review of TiO2 NTs on Ti metal: Electrochemical synthesis, functionalization and potential use as bone implants. Mater. Sci. Eng. C 2017, 76, 1401–1412. [Google Scholar] [CrossRef] [PubMed]
- Tranquilli Leali, P.; Merolli, A. Fundamentals of Biomaterials. In Biomaterials in Hand Surgery; Merolli, A., Joyce, T.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2009; pp. 1–11. [Google Scholar]
- Frandsen, C.J.; Noh, K.; Brammer, K.S.; Johnston, G.; Jin, S. Hybrid micro/nano-topography of a TiO2 nanotube-coated commercial zirconia femoral knee implant promotes bone cell adhesion in vitro. Mater. Sci. Eng. C 2013, 33, 2752–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minagar, S.; Wang, J.; Berndt, C.C.; Ivanova, E.P.; Wen, C. Cell response of anodized nanotubes on titanium and titanium alloys. J. Biomed. Mater. Res. Part. A 2013, 101 A, 2726–2739. [Google Scholar] [CrossRef]
- Salata, O.V. Applications of nanoparticles in biology and medicine. J. Nanobiotechnol. 2004, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Baldwin, F.; Craig, T.J.; Shiel, A.I.; Cox, T.; Lee, K.; Mansell, J.P. Polydopamine-lysophosphatidate-functionalised titanium: A novel hybrid surface finish for bone regenerative applications. Molecules 2020, 25, 1583. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.Y.; Kim, Y.K.; Park, I.S.; Jin, G.C.; Bae, T.S.; Lee, M.H. Effect of alkali and heat treatments for bioactivity of TiO2 nanotubes. Appl. Surf. Sci. 2014, 321, 412–419. [Google Scholar] [CrossRef]
- Oh, S.H.; Finõnes, R.R.; Daraio, C.; Chen, L.H.; Jin, S. Growth of nano-scale hydroxyapatite using chemically treated titanium oxide nanotubes. Biomaterials 2005, 26, 4938–4943. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Jin, S. Titanium oxide nanotubes with controlled morphology for enhanced bone growth. Mater. Sci. Eng. C 2006, 26, 1301–1306. [Google Scholar] [CrossRef]
- Yao, C.; Webster, T.J. Prolonged antibiotic delivery from anodized nanotubular titanium using a co-precipitation drug loading method. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2009, 91, 587–595. [Google Scholar] [CrossRef]
- Pawlik, A.; Socha, R.P.; Hubalek Kalbacova, M.; Sulka, G.D. Surface modification of nanoporous anodic titanium dioxide layers for drug delivery systems and enhanced SAOS-2 cell response. Colloids Surf. B Biointerfaces 2018, 171, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Anselme, K.; Ploux, L.; Ponche, A. Cell/material interfaces: Influence of surface chemistry and surface topography on cell adhesion. J. Adhes. Sci. Technol. 2010, 24, 831–852. [Google Scholar] [CrossRef]
- Mani, G.; Johnson, D.M.; Marton, D.; Dougherty, V.L.; Feldman, M.D.; Patel, D.; Ayon, A.A.; Mauli Agrawal, C. Stability of self-assembled monolayers on titanium and gold. Langmuir 2008, 24, 6774–6784. [Google Scholar] [CrossRef] [PubMed]
- Brnardić, I.; Huskić, M.; Umek, P.; Grgurić, T.H. Sol-gel functionalization of sodium TiO2 nanotubes and nanoribbons with aminosilane molecules. Ceram. Int. 2013, 39, 9459–9464. [Google Scholar] [CrossRef]
- Huskić, M.; Grgurić, T.H.; Umek, P.; Brnardić, I. Functionalization of sodium titanate nanoribbons with silanes and their use in the reinforcement of epoxy nanocomposites. Polym. Compos. 2013, 34, 1382–1388. [Google Scholar] [CrossRef]
- Mandal, S.S.; Jose, D.; Bhattacharyya, A.J. Role of surface chemistry in modulating drug release kinetics in titania nanotubes. Mater. Chem. Phys. 2014, 147, 247–253. [Google Scholar] [CrossRef]
- Lin, S.P.; Huang, S.Y.; Chen, S.F.; Vinzons, L.U.; Ciou, J.Y.; Wong, P.J. Investigation of the interfacial effects of small chemical-modified TiO2 nanotubes on 3T3 fibroblast responses. ACS Appl. Mater. Interfaces 2014, 6, 12071–12082. [Google Scholar] [CrossRef]
- Paz, Y. Self-assembled monolayers and titanium dioxide: From surface patterning to potential applications. Beilstein J. Nanotechnol. 2011, 2, 845–861. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, M.; Mazare, A.; Gongadze, E.; Perutkova, S.; Kralj-Iglic, V.; Milošev, I.; Schmuki, P.; Iglič, A.; Mozetič, M. Titanium nanostructures for biomedical applications. Nanotechnology 2015, 26, 062002. [Google Scholar] [CrossRef] [PubMed]
- Jarosz, M.; Syrek, K.; Kapusta-Kołodziej, J.; Mech, J.; Małek, K.; Hnida, K.; Łojewski, T.; Jaskuła, M.; Sulka, G.D. Heat treatment effect on crystalline structure and photoelectrochemical properties of anodic TiO2 nanotube arrays formed in ethylene glycol and glycerol based electrolytes. J. Phys. Chem. C 2015, 119, 24182–24191. [Google Scholar] [CrossRef]
- Sreekantan, S.; Saharudin, K.A.; Wei, L.C. Formation of TiO2 nanotubes via anodization and potential applications for photocatalysts, biomedical materials, and photoelectrochemical cell. IOP Conf. Ser. Mater. Sci. Eng. 2011, 21, 012002. [Google Scholar] [CrossRef]
- Cipriano, A.F.; Miller, C.; Liu, H. Anodic growth and biomedical applications of TiO2 nanotubes. J. Biomed. Nanotechnol. 2014, 10, 2977–3003. [Google Scholar] [CrossRef]
- Ercan, B.; Taylor, E.; Alpaslan, E.; Webster, T.J. Diameter of titanium nanotubes influences anti-bacterial efficacy. Nanotechnology 2011, 22, 295102. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Cui, Q.; Feng, B.; Wang, J.; Lu, X.; Weng, J. Antibacterial activity of TiO2 nanotubes: Influence of crystal phase, morphology and Ag deposition. Appl. Surf. Sci. 2013, 284, 179–183. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Szuwarzyński, M.; Jaskuła, M.; Sulka, G.D. Nanoporous anodic titanium dioxide layers as potential drug delivery systems: Drug release kinetics and mechanism. Colloids Surf. B Biointerfaces 2016, 143, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Jarosz, M.; Syrek, K.; Sulka, G.D. Co-delivery of ibuprofen and gentamicin from nanoporous anodic titanium dioxide layers. Colloids Surf. B Biointerfaces 2017, 152, 95–102. [Google Scholar] [CrossRef]
- Popat, K.C.; Eltgroth, M.; LaTempa, T.J.; Grimes, C.A.; Desai, T.A. Titania nanotubes: A novel platform for drug-eluting coatings for medical implants? Small 2007, 3, 1878–1881. [Google Scholar] [CrossRef] [PubMed]
- Doadrio, A.L.; Sánchez-Montero, J.M.; Doadrio, J.C.; Salinas, A.J.; Vallet-Regí, M. A molecular model to explain the controlled release from SBA-15 functionalized with APTES. Microporous Mesoporous Mater. 2014, 195, 43–49. [Google Scholar] [CrossRef] [Green Version]
- Doadrio, A.L.; Conde, A.; Arenas, M.A.; Hernández-López, J.M.; De Damborenea, J.J.; Pérez-Jorge, C.; Esteban, J.; Vallet-Regí, M. Use of anodized titanium alloy as drug carrier: Ibuprofen as model of drug releasing. Int. J. Pharm. 2015, 492, 207–212. [Google Scholar] [CrossRef]
- Brammer, K.S.; Frandsen, C.J.; Jin, S. TiO2 nanotubes for bone regeneration. Trends Biotechnol. 2012, 30, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Mondrinos, M.J.; Chen, X.; Gandhi, M.R.; Ko, F.K.; Lelkes, P.I. Elastin Blends for Tissue Engineering Scaffolds. J. Biomed. Mater. Res. Part. A 2006, 79, 963–973. [Google Scholar] [CrossRef]
- Czekanska, E.M.; Stoddart, M.J.; Richards, R.G.; Hayes, J.S. In search of an osteoblast cell model for in vitro research. Eur. Cells Mater. 2012, 24, 1–17. [Google Scholar] [CrossRef]
- Lai, M.; Jin, Z.; Su, Z. Surface modification of TiO2 nanotubes with osteogenic growth peptide to enhance osteoblast differentiation. Mater. Sci. Eng. C 2017, 73, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.; Jin, Z.; Qiao, W. Surface immobilization of gelatin onto TiO2 nanotubes to modulate osteoblast behavior. Colloids Surf. B Biointerfaces 2017, 159, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Patil-Sen, Y.; Junkar, I.; Kulkarni, C.V.; Lorenzetti, M.; Iglič, A. Wettability studies of topologically distinct titanium surfaces. Colloids Surf. B Biointerfaces 2015, 129, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Gongadze, E.; Kabaso, D.; Bauer, S.; Slivnik, T.; Schmuki, P.; van Rienen, U.; Iglič, A. Adhesion of osteoblasts to a nanorough titanium implant surface. Int. J. Nanomed. 2011, 6, 1801–1816. [Google Scholar]
- Yang, T.; Qian, S.; Qiao, Y.; Liu, X. Cytocompatibility and antibacterial activity of titania nanotubes incorporated with gold nanoparticles. Colloids Surf. B Biointerfaces 2016, 145, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Brammer, K.S.; Oh, S.; Cobb, C.J.; Bjursten, L.M.; van der Heyde, H.; Jin, S. Improved bone-forming functionality on diameter-controlled TiO2 nanotube surface. Acta Biomater. 2009, 5, 3215–3223. [Google Scholar] [CrossRef] [PubMed]
- YazdanYar, A.; Aschauer, U.; Bowen, P. Interaction of biologically relevant ions and organic molecules with titanium oxide (rutile) surfaces: A review on molecular dynamics studies. Colloids Surf. B Biointerfaces 2018, 161, 563–577. [Google Scholar] [CrossRef]
- He, J.; Zhou, W.; Zhou, X.; Zhong, X.; Zhang, X.; Wan, P.; Zhu, B.; Chen, W. The anatase phase of nanotopography titania plays an important role on osteoblast cell morphology and proliferation. J. Mater. Sci. Mater. Med. 2008, 19, 3465–3472. [Google Scholar] [CrossRef]
- Kunze, J.; Müller, L.; Macak, J.M.; Greil, P.; Schmuki, P.; Müller, F.A. Time-dependent growth of biomimetic apatite on anodic TiO2 nanotubes. Electrochim. Acta 2008, 53, 6995–7003. [Google Scholar] [CrossRef]
- Morgado, E.; De Abreu, M.A.S.; Moure, G.T.; Marinkovic, B.A.; Jardim, P.M.; Araujo, A.S. Characterization of nanostructured titanates obtained by alkali treatment of TiO2-anatases with distinct crystal sizes. Chem. Mater. 2007, 19, 665–676. [Google Scholar] [CrossRef]
- Wang, Y.; Wen, C.; Hodgson, P.; Li, Y. Biocompatibility of TiO2 nanotubes with different topographies. J. Biomed. Mater. Res. Part. A 2014, 102, 743–751. [Google Scholar] [CrossRef]
- Shin, D.H.; Shokuhfar, T. Wettability changes of TiO2 nanotube surfaces. Nanotechnology 2011, 22, 315704. [Google Scholar] [CrossRef] [PubMed]
- Kao, L.; Chen, Y. Characterization, photoelectrochemical properties, and surface wettabilities of transparent porous TiO2 thin films. J. Photochem. Photobiol. A Chem. 2017, 340, 109–119. [Google Scholar] [CrossRef]
- Hamlekhan, A.; Butt, A.; Patel, S.; Royhman, D.; Takoudis, C.; Sukotjo, C.; Yuan, J.; Jursich, G.; Mathew, M.T.; Hendrickson, W.; et al. Fabrication of Anti-Aging TiO2 Nanotubes on Biomedical Ti Alloys. PLoS ONE 2014, 9, e96213. [Google Scholar] [CrossRef]
- Pautke, C.; Schieker, M.; Tischer, T.; Kolk, A.; Neth, P.; Mutschler, W.; Milz, S. Characterization of osteosarcoma cell lines MG-63, Saos-2 and U-2 OS in comparison to human osteoblasts. Anticancer Res. 2004, 24, 3743–3748. [Google Scholar] [PubMed]
- Khoshnood, N.; Zamanian, A.; Massoudi, A. Effect of silane-coupling modification on bioactivity and in vitro properties of anodized titania nanotube arrays. Mater. Lett. 2016, 185, 374–378. [Google Scholar] [CrossRef]
- Dalby, M.J. Cellular response to low adhesion nanotopographies. Int. J. Nanomed. 2007, 2, 373–381. [Google Scholar]
- Jarosz, M.; Kapusta-Kołodziej, J.; Jaskuła, M.; Sulka, G.D. Effect of different polishing methods on anodic titanium dioxide formation. J. Nanomater. 2015, 2015, 295126. [Google Scholar] [CrossRef] [Green Version]
- Sulka, G.D.; Kapusta-Kołodziej, J.; Brzózka, A.; Jaskuła, M. Fabrication of nanoporous TiO2 by electrochemical anodization. Electrochim. Acta 2010, 55, 4359–4367. [Google Scholar] [CrossRef]
- Jarosz, M.; Pawlik, A.; Kapusta-Kołodziej, J.; Jaskuła, M.; Sulka, G.D. Effect of the previous usage of electrolyte on growth of anodic titanium dioxide (ATO) in a glycerol-based electrolyte. Electrochim. Acta 2014, 136, 412–421. [Google Scholar] [CrossRef]
- Kapusta-Kołodziej, J.; Tynkevych, O.; Pawlik, A.; Jarosz, M.; Mech, J.; Sulka, G.D. Electrochemical growth of porous titanium dioxide in a glycerol-based electrolyte at different temperatures. Electrochim. Acta 2014, 144, 127–135. [Google Scholar] [CrossRef]
- R Studio Software. Available online: www.rstudio.com (accessed on 3 July 2018).
- Chissoe, W.F.; Vezey, E.L.; Skvarla, J.J. Hexamethyldisilazane as a drying agent for pollen scanning electron microscopy. Biotech. Histochem. 1994, 69, 192–198. [Google Scholar] [CrossRef]






| Sample | Atomic Content [%] | |||||||
|---|---|---|---|---|---|---|---|---|
| C | O | Ti | F | Na | Si | N | S | |
| an | 11.14 | 63.70 | 24.61 | 0.55 | - | - | - | - |
| Nan | 9.12 | 59.28 | 19.05 | 0.72 | 11.84 | - | - | - |
| ANan | 19.72 | 50.75 | 15.85 | 0.00 | 4.32 | 6.29 | 3.07 | - |
| GNan | 19.03 | 54.49 | 16.94 | 0.18 | 3.89 | 5.47 | - | - |
| MNan | 14.57 | 55.07 | 17.68 | 0.44 | 4.62 | 4.08 | - | 3.53 |
| Sample | Contact Angle [°] |
|---|---|
| an | 41.1 ± 3.3 |
| Nan | 14.3 ± 2.2 |
| ANan | 20.3 ± 2.7 |
| GNan | 18.6 ± 3.9 |
| MNan | 29.1 ± 3.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pawlik, A.; Jarosz, M.; Socha, R.P.; Sulka, G.D. The Impacts of Crystalline Structure and Different Surface Functional Groups on Drug Release and the Osseointegration Process of Nanostructured TiO2. Molecules 2021, 26, 1723. https://doi.org/10.3390/molecules26061723
Pawlik A, Jarosz M, Socha RP, Sulka GD. The Impacts of Crystalline Structure and Different Surface Functional Groups on Drug Release and the Osseointegration Process of Nanostructured TiO2. Molecules. 2021; 26(6):1723. https://doi.org/10.3390/molecules26061723
Chicago/Turabian StylePawlik, Anna, Magdalena Jarosz, Robert P. Socha, and Grzegorz D. Sulka. 2021. "The Impacts of Crystalline Structure and Different Surface Functional Groups on Drug Release and the Osseointegration Process of Nanostructured TiO2" Molecules 26, no. 6: 1723. https://doi.org/10.3390/molecules26061723
APA StylePawlik, A., Jarosz, M., Socha, R. P., & Sulka, G. D. (2021). The Impacts of Crystalline Structure and Different Surface Functional Groups on Drug Release and the Osseointegration Process of Nanostructured TiO2. Molecules, 26(6), 1723. https://doi.org/10.3390/molecules26061723