Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons
Abstract
:1. Introduction
2. Origin of π-Holes
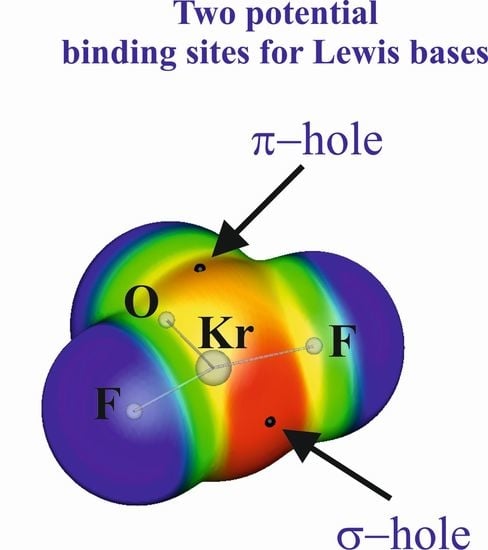
3. σ-Hole and π-Hole on the Same Atom
4. σ-Hole and π-Hole on Different Atoms
4.1. Combination of σ-Hole on Halogen or Chalcogen with π-hole on Aromatic Ring
| Molecule | Hole Source | Hole Type | VS,max | References |
|---|---|---|---|---|
| 1,4-DITFB | I | σ | 32.3 | [140] |
| Carbon/Benzene ring | π | 15.1 | [140] | |
| C6H5Br | Br | σ | up to ~15 | [142] |
| Benzene Ring | π | up to ~15 | [142] | |
| TITFB | I | σ | 30.1 | [143] |
| Carbon/Benzene Ring | π | 11.4 | [143] | |
| Halogen Substituted 1,3,4-oxadiazol-2IJ3H)-thiones | S (Chalcogen), Cl, Br | σ | up to ~37 | [144] |
| Oxadiazole and Benzyl Ring | π | up to ~37 | [144] | |
| Haloperfluorobenzene | Cl, Br, I | σ | 20.9 to 32.8 | [145] |
| Benzene Ring | π | 12.6 to 19.8 | [145] | |
| XC3H4N2+; X = F, Cl, Br, I | Cl, Br, I | σ | 105.9 to 117.5 | [146] |
| Imidazolium Ring | π | 111.0 to 127.9 | [146] |
4.2. Combination of σ-Hole Halogen Bond with Other π-Hole Sources
4.3. Other Examples
5. Conclusions and Prospective
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clark, T.; Hennemann, M.; Murray, J.S.; Politzer, P. Halogen bonding: The σ-hole: Proceedings of “Modeling interactions in biomolecules II”, Prague, September 5th–9th, 2005. J. Mol. Model. 2007, 13, 291–296. [Google Scholar] [CrossRef]
- Guthrie, F. On the Iodide of Iodammonium. J. Chem. Soc. 1863, 16, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Remsen, I.; Norris, J.F. Action of the halogens on the methyl-amines. Am. Chem. J. 1896, 18, 90–96. [Google Scholar]
- Hope, H.; McCullough, J.D. The crystal structure of the molecular complex of iodine with tetrahydroselenophene, C4H8Se.I2. Acta Cryst. 1964, 17, 712–718. [Google Scholar] [CrossRef]
- Olie, K.; Mijlhoff, F.C. The crystal structure of POBr3 and intermolecular bonding. Acta Cryst. Sect. B 1969, 25, 974–977. [Google Scholar] [CrossRef]
- Brinck, T.; Murray, J.S.; Politzer, P. Surface electrostatic potentials of halogenated methanes as indicators of directional intermolecular interactions. Int. J. Quantum Chem. 1992, 44, 57–64. [Google Scholar] [CrossRef]
- Murray, J.S.; Lane, P.; Clark, T.; Politzer, P. Sigma-hole bonding: Molecules containing group VI atoms. J. Mol. Model. 2007, 13, 1033–1038. [Google Scholar] [CrossRef]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.G.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Concha, M.C. Sigma-hole bonding between like atoms; a fallacy of atomic charges. J. Mol. Model. 2008, 14, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Lane, P.; Politzer, P. Expansion of the sigma-hole concept. J. Mol. Model. 2009, 15, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. Halogen bonding: An electrostatically-driven highly directional noncovalent interaction. Phys. Chem. Chem. Phys. 2010, 12, 7748–7757. [Google Scholar] [CrossRef] [PubMed]
- Murray, J.S.; Politzer, P. The electrostatic potential: An overview. Wires Comput. Mol. Sci. 2011, 1, 153–163. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Interaction and polarization energy relationships in σ-hole and π-hole bonding. Crystals 2020, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T. sigma-Hole Bonding: A Physical Interpretation. Top Curr. Chem. 2015, 358, 19–42. [Google Scholar] [PubMed]
- Politzer, P.; Murray, J.S.; Clark, T.; Resnati, G. The sigma-hole revisited. Phys. Chem. Chem. Phys. 2017, 19, 32166–32178. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Electrostatics and Polarization in sigma- and pi-Hole Noncovalent Interactions: An Overview. ChemPhysChem 2020, 21, 579–588. [Google Scholar] [CrossRef]
- Azofra, L.M.; Alkorta, I.; Scheiner, S. Chalcogen bonds in complexes of SOXY (X, Y = F, Cl) with nitrogen bases. J. Phys. Chem. A 2015, 119, 535–541. [Google Scholar] [CrossRef] [Green Version]
- Vincent De Paul, N.N.; Scheiner, S. Chalcogen bonding between tetravalent SF4 and amines. J. Phys. Chem. A 2014, 118, 10849–10856. [Google Scholar]
- Alikhani, E.; Fuster, F.; Madebene, B.; Grabowski, S.J. Topological reaction sites-very strong chalcogen bonds. Phys. Chem. Chem. Phys. 2014, 16, 2430–2442. [Google Scholar] [CrossRef]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen bond: A sister noncovalent bond to halogen bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef]
- de Azevedo Santos, L.; Ramalho, T.C.; Hamlin, T.A.; Bickelhaupt, F.M. Chalcogen bonds: Hierarchical ab initio benchmark and density functional theory performance study. J. Comput. Chem. 2021, 42, 688–698. [Google Scholar] [CrossRef]
- de Azevedo Santos, L.; van der Lubbe, S.C.C.; Hamlin, T.A.; Ramalho, T.C.; Matthias Bickelhaupt, F. A Quantitative Molecular Orbital Perspective of the Chalcogen Bond. ChemistryOpen 2021. [Google Scholar] [CrossRef]
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Pnicogen Bonded Complexes of PO2X (X = F, Cl) with Nitrogen Bases. J. Phys. Chem. A 2013, 117, 10497–10503. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguero, J. Properties of Complexes H2C=(X)P:PXH2, for X = F, Cl, OH, CN, NC, CCH, H, CH3, and BH2: P center dot center dot center dot P Pnicogen Bonding at sigma-Holes and pi-Holes. J. Phys. Chem. A 2013, 117, 11592–11604. [Google Scholar] [CrossRef]
- Scheiner, S. Detailed comparison of the pnicogen bond with chalcogen, halogen, and hydrogen bonds. Int. J. Quantum Chem. 2013, 113, 1609–1620. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. The pnicogen bond: Its relation to hydrogen, halogen, and other noncovalent bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Guan, L.Y.; Mo, Y.R. Electron Transfer in Pnicogen Bonds. J. Phys. Chem. A 2014, 118, 8911–8921. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel-Bonding Interaction: Rediscovered Supramolecular Force? Angew. Chem. Int. Ed. 2013, 52, 12317–12321. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Tetrel Bonding Interactions. Chem. Rec. 2016, 16, 473–487. [Google Scholar] [CrossRef]
- Marin-Luna, M.; Alkorta, I.; Elguero, J. Cooperativity in Tetrel Bonds. J. Phys. Chem. A 2016, 120, 648–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desiraju, G.R.; Shing Ho, P.; Kloo, L.; Legon, A.C.; Marquardt, R.; Metrangolo, P.; Politzer, P.; Resnati, G.; Rissanen, K. Definition of the halogen bond (IUPAC recommendations 2013). Pure Appl. Chem. 2013, 85, 1711–1713. [Google Scholar] [CrossRef]
- Aakeroy, C.B.; Bryce, D.L.; Desiraju, G.; Frontera, A.; Legon, A.C.; Nicotra, F.; Rissanen, K.; Scheiner, S.; Terraneo, G.; Metrangolo, P.; et al. Definition of the chalcogen bond (IUPAC Recommendations 2019). Pure Appl. Chem. 2019, 91, 1889–1892. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Afkhami, F.A.; Zangrando, E.; Kaminsky, W.; Frontera, A.; Safin, D.A. A supramolecular 3D structure constructed from a new metal chelate self-assembled from Sn(NCS)(2) and phenyl(pyridin-2-yl)methylenepicolinohydrazide. J. Mol. Struct. 2021, 1224, 129188. [Google Scholar] [CrossRef]
- Zhang, J.H.; Su, Z.Z.; Luo, J.X.; Zhao, Y.; Wang, H.G.; Ying, S.M. Synthesis, structure, and characterization of a mixed amines thiogermanate [NH4](2)[NH2(CH3)(2)](2)Ge2S6. Polyhedron 2020, 182, 114486. [Google Scholar] [CrossRef]
- Zelenkov, L.E.; Ivanov, D.M.; Sadykov, E.K.; Bokach, N.A.; Galmes, B.; Frontera, A.; Kukushkin, V.Y. Semicoordination Bond Breaking and Halogen Bond Making Change the Supramolecular Architecture of Metal-Containing Aggregates. Cryst. Growth Des. 2020, 20, 6956–6965. [Google Scholar] [CrossRef]
- Yusof, E.N.M.; Latif, M.A.M.; Tahir, M.I.M.; Sakoff, J.A.; Veerakumarasivam, A.; Page, A.J.; Tiekink, E.R.T.; Ravoof, T.B.S.A. Homoleptic tin(IV) compounds containing tridentate ONS dithiocarbazate Schiff bases: Synthesis, X-ray crystallography, DFT and cytotoxicity studies. J. Mol. Struct. 2020, 1205, 127635. [Google Scholar] [CrossRef]
- Walton, I.; Chen, C.; Rimsza, J.M.; Nenoff, T.M.; Walton, K.S. Enhanced Sulfur Dioxide Adsorption in UiO-66 Through Crystal Engineering and Chalcogen Bonding. Cryst. Growth Des. 2020, 20, 6139–6146. [Google Scholar] [CrossRef]
- Venosova, B.; Koziskova, J.; Kozisek, J.; Herich, P.; Luspai, K.; Petricek, V.; Hartung, J.; Muller, M.; Hubschle, C.B.; van Smaalen, S.; et al. Charge density of 4-methyl-3-[(tetrahydro-2H-pyran-2-yl)oxy]thiazole-2(3H)-thione. A comprehensive multipole refinement, maximum entropy method and density functional theory study. Acta Cryst. B 2020, 76, 450–468. [Google Scholar] [CrossRef]
- Villasenor-Granados, T.O.; Montes-Tolentino, P.; Rodriguez-Lopez, G.; Sanchez-Ruiz, S.A.; Flores-Parra, A. Structural analysis of tris(5-methyl- [1,3,5]-dithiazinan-2-yl)stibine, its reactions with chalcogens. Intramolecular chalcogen-bonding interactions. J. Mol. Struct. 2020, 1200, 127050. [Google Scholar] [CrossRef]
- Kumar, V.; Xu, Y.J.; Bryce, D.L. Double Chalcogen Bonds: Crystal Engineering Stratagems via Diffraction and Multinuclear Solid-State Magnetic Resonance Spectroscopy. Chem. Eur. J. 2020, 26, 3275–3286. [Google Scholar] [CrossRef]
- Fourmigue, M.; Dhaka, A. Chalcogen bonding in crystalline diselenides and selenocyanates: From molecules of pharmaceutical interest to conducting materials. Coord. Chem. Rev. 2020, 403, 213084. [Google Scholar] [CrossRef]
- Michalczyk, M.; Malik, M.; Zierkiewicz, W.; Scheiner, S. Experimental and Theoretical Studies of Dimers Stabilized by Two Chalcogen Bonds in the Presence of a N···N Pnicogen Bond. J. Phys. Chem. A 2021, 125, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Kinzhalov, M.A.; Popova, E.A.; Petrov, M.L.; Khoroshilova, O.V.; Mahmudov, K.T.; Pombeiro, A.J.L. Pnicogen and chalcogen bonds in cyclometalated iridium(III) complexes. Inorg. Chim. Acta 2018, 477, 31–33. [Google Scholar] [CrossRef]
- Zapata, F.; Gonzalez, L.; Bastida, A.; Bautista, D.; Caballero, A. Formation of self-assembled supramolecular polymers by anti-electrostatic anion-anion and halogen bonding interactions. Chem. Commun. 2020, 56, 7084–7087. [Google Scholar] [CrossRef] [PubMed]
- Soldatova, N.S.; Postnikov, P.S.; Suslonov, V.V.; Kissler, T.Y.; Ivanov, D.M.; Yusubov, M.S.; Galmes, B.; Frontera, A.; Kukushkin, V.Y. Diaryliodonium as a double sigma-hole donor: The dichotomy of thiocyanate halogen bonding provides divergent solid state arylation by diaryliodonium cations. Org. Chem. Front. 2020, 7, 2230–2242. [Google Scholar] [CrossRef]
- Scilabra, P.; Terraneo, G.; Daolio, A.; Baggioli, A.; Famulari, A.; Leroy, C.; Bryce, D.L.; Resnati, G. 4,4 ‘-Dipyridyl Dioxide center dot SbF3 Cocrystal: Pnictogen Bond Prevails over Halogen and Hydrogen Bonds in Driving Self-Assembly. Cryst. Growth Des. 2020, 20, 916–922. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, M.A.; Heyer, E.; Less, R.J.; Pask, C.M.; Arauzo, A.; Campo, J.; Rawson, J.M. An Investigation of Halogen Bonding as a Structure-Directing Interaction in Dithiadiazolyl Radicals. Cryst. Growth Des. 2020, 20, 4313–4324. [Google Scholar] [CrossRef]
- Pramanik, S.; Chopra, D. Unravelling the Importance of H bonds, σ–hole and π–hole-Directed Intermolecular Interactions in Nature. J. Indian I Sci. 2020, 100, 43–59. [Google Scholar] [CrossRef]
- Orlova, A.P.; Jasien, P.G. Halogen bonding in self-assembling systems: A comparison of intra- and interchain binding energies. Comput. Chem. 2018, 1139, 63–69. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. A Survey of Supramolecular Aggregation Based on Main Group Element Selenium Secondary Bonding Interactions—A Survey of the Crystallographic Literature. Crystals 2020, 10, 503. [Google Scholar] [CrossRef]
- Seth, S.K.; Bauzá, A.; Frontera, A. Chapter 9 Quantitative Analysis of Weak Non-covalent σ-Hole and π-Hole Interactions. In Monogr Supramol Chem; The Royal Society of Chemistry: London, UK, 2019; pp. 285–333. [Google Scholar]
- Taylor, M.S. Anion recognition based on halogen, chalcogen, pnictogen and tetrel bonding. Coord. Chem. Rev. 2020, 413, 213270. [Google Scholar] [CrossRef]
- Navarro-Garcia, E.; Galmes, B.; Velasco, M.D.; Frontera, A.; Caballero, A. Anion Recognition by Neutral Chalcogen Bonding Receptors: Experimental and Theoretical Investigations. Chem. Eur. J. 2020, 26, 4706–4713. [Google Scholar] [CrossRef] [PubMed]
- Borissov, A.; Marques, I.; Lim, J.Y.C.; Felix, V.; Smith, M.D.; Beer, P.D. Anion Recognition in Water by Charge-Neutral Halogen and Chalcogen Bonding Foldamer Receptors. J. Am. Chem. Soc. 2019, 141, 4119–4129. [Google Scholar] [CrossRef] [PubMed]
- Semenov, N.A.; Gorbunov, D.E.; Shakhova, M.V.; Salnikov, G.E.; Bagryanskaya, I.Y.; Korolev, V.V.; Beckmann, J.; Gritsan, N.P.; Zibarev, A.V. Donor-Acceptor Complexes between 1,2,5-Chalcogenadiazoles (Te, Se, S) and the Pseudohalides CN- and XCN- (X = O, S, Se, Te). Chem. Eur. J. 2018, 24, 12983–12991. [Google Scholar] [CrossRef]
- Naseer, M.M.; Hussain, M.; Bauza, A.; Lo, K.M.; Frontera, A. Intramolecular Noncovalent Carbon Bonding Interaction Stabilizes the cis Conformation in Acylhydrazones. Chempluschem 2018, 83, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.C.; Beer, P.D. Sigma-Hole Interactions in Anion Recognition. Chem-Us 2018, 4, 731–783. [Google Scholar] [CrossRef]
- Liu, Y.-Z.; Yuan, K.; Liu, L.; Yuan, Z.; Zhu, Y.-C. Anion Recognition Based on a Combination of Double-Dentate Hydrogen Bond and Double-Side Anion−π Noncovalent Interactions. J. Phys. Chem. A 2017, 121, 892–900. [Google Scholar] [CrossRef]
- Lim, J.Y.C.; Marques, I.; Thompson, A.L.; Christensen, K.E.; Felix, V.; Beer, P.D. Chalcogen Bonding Macrocycles and [2]Rotaxanes for Anion Recognition. J. Am. Chem. Soc. 2017, 139, 3122–3133. [Google Scholar] [CrossRef]
- Scheiner, S. Tetrel Bonding as a Vehicle for Strong and Selective Anion Binding. Molecules 2018, 23, 1147. [Google Scholar] [CrossRef] [Green Version]
- Kociok-Kohn, G.; Mahon, M.F.; Molloy, K.C.; Price, G.J.; Prior, T.J.; Smith, D.R.G. Biomimetic polyorganosiloxanes: Model compounds for new materials. Dalton Trans. 2014, 43, 7734–7746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politzer, P.; Murray, J.S.; Concha, M.C. Halogen bonding and the design of new materials: Organic bromides, chlorides and perhaps even fluorides as donors. J. Mol. Model. 2007, 13, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Radovic, L.R. Probing the ‘elephant’: On the essential difference between graphenes and polycyclic aromatic hydrocarbons. Carbon 2021, 171, 798–805. [Google Scholar] [CrossRef]
- Maruthapandi, M.; Eswaran, L.; Cohen, R.; Perkas, N.; Luong, J.H.T.; Gedanken, A. Silica-Supported Nitrogen-Enriched Porous Benzimidazole-Linked and Triazine-Based Polymers for the Adsorption of CO2. Langmuir 2020, 36, 4280–4288. [Google Scholar] [CrossRef] [PubMed]
- Kar, I.; Chatterjee, J.; Harnagea, L.; Kushnirenko, Y.; Fedorov, A.V.; Shrivastava, D.; Buchner, B.; Mahadevan, P.; Thirupathaiah, S. Metal-chalcogen bond-length induced electronic phase transition from semiconductor to topological semimetal in ZrX2 (X = Se and Te). Phys. Rev. B 2020, 101, 165122. [Google Scholar] [CrossRef] [Green Version]
- Isaeva, A.; Ruck, M. Crystal Chemistry and Bonding Patterns of Bismuth-Based Topological Insulators. Inorg. Chem. 2020, 59, 3437–3451. [Google Scholar] [CrossRef] [PubMed]
- Ho, P.C.; Wang, J.Z.; Meloni, F.; Vargas-Baca, I. Chalcogen bonding in materials chemistry. Coord. Chem. Rev. 2020, 422, 213464. [Google Scholar] [CrossRef]
- Daniels, C.L.; Knobeloch, M.; Yox, P.; Adamson, M.A.S.; Chen, Y.H.; Dorn, R.W.; Wu, H.; Zhou, G.Q.; Fan, H.J.; Rossini, A.J.; et al. Intermetallic Nanocatalysts from Heterobimetallic Group 10-14 Pyridine-2-thiolate Precursors. Organometallics 2020, 39, 1092–1104. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, W.Z.; Wang, Y.B. Tetrel bonding on graphene. Comput. Chem. 2019, 1147, 8–12. [Google Scholar] [CrossRef]
- Sung, S.H.; Schnitzer, N.; Brown, L.; Park, J.; Hovden, R. Stacking, strain, and twist in 2D materials quantified by 3D electron diffraction. Phys. Rev. Mater. 2019, 3, 064003. [Google Scholar] [CrossRef] [Green Version]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen bonding in synthesis, catalysis and design of materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [Green Version]
- Pina, M.D.N.; Frontera, A.; Bauza, A. Quantifying Intramolecular Halogen Bonds in Nucleic Acids: A Combined Protein Data Bank and Theoretical Study. Acs Chem. Biol. 2020, 15, 1942–1948. [Google Scholar] [CrossRef]
- Lundemba, A.S.; Bibelayi, D.D.; Wood, P.A.; Pradon, J.; Yav, Z.G. sigma-Hole interactions in small-molecule compounds containing divalent sulfur groups R-1-S-R-2. Acta Cryst. B 2020, 76, 707–718. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. Differential Binding of Tetrel-Bonding Bipodal Receptors to Monatomic and Polyatomic Anions. Molecules 2019, 24, 227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riel, A.M.S.; Huynh, H.T.; Jeannin, O.; Berryman, O.; Fourmigue, M. Organic Selenocyanates as Halide Receptors: From Chelation to One-Dimensional Systems. Cryst. Growth Des. 2019, 19, 1418–1425. [Google Scholar] [CrossRef]
- Michalczyk, M.; Zierkiewicz, W.; Wysokinski, R.; Scheiner, S. Theoretical Studies of IR and NMR Spectral Changes Induced by Sigma-Hole Hydrogen, Halogen, Chalcogen, Pnicogen, and Tetrel Bonds in a Model Protein Environment. Molecules 2019, 24, 3329. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Li, Q.; Scheiner, S. The pi-Tetrel Bond and its Influence on Hydrogen Bonding and Proton Transfer. Chemphyschem 2018, 19, 736–743. [Google Scholar] [CrossRef]
- Trievel, R.C.; Scheiner, S. Crystallographic and Computational Characterization of Methyl Tetrel Bonding in S-Adenosylmethionine-Dependent Methyltransferases. Molecules 2018, 23, 2965. [Google Scholar] [CrossRef] [Green Version]
- Scheiner, S. Halogen Bonds Formed between Substituted Imidazoliums and N Bases of Varying N-Hybridization. Molecules 2017, 22, 1634. [Google Scholar] [CrossRef]
- Montaña, Á.M. The σ and π Holes. The Halogen and Tetrel Bondings: Their Nature, Importance and Chemical, Biological and Medicinal Implications. Chemistryselect 2017, 2, 9094–9112. [Google Scholar] [CrossRef]
- Grabowski, S.J. Tetrel bond-sigma-hole bond as a preliminary stage of the S(N)2 reaction. Phys. Chem. Chem. Phys. 2014, 16, 1824–1834. [Google Scholar] [CrossRef]
- Angarov, V.; Kozuch, S. On the σ, π and δ hole interactions: A molecular orbital overview. New J. Chem. 2018, 42, 1413–1422. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional sigma/-Hole Interactions. Chemphyschem 2015, 16, 2496–2517. [Google Scholar] [CrossRef]
- Gao, L.; Zeng, Y.; Zhang, X.; Meng, L. Comparative studies on group III sigma-hole and pi-hole interactions. J. Comput. Chem. 2016, 37, 1321–1327. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. Directionality of pi-holes in nitro compounds. Chem. Commun. 2015, 51, 1491–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murray, J.S.; Lane, P.; Clark, T.; Riley, K.E.; Politzer, P. sigma-Holes, pi-holes and electrostatically-driven interactions. J. Mol. Model. 2012, 18, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, S.J. The Nature of Triel Bonds, a Case of B and Al Centres Bonded with Electron Rich Sites. Molecules 2020, 25, 2703. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel bond and coordination of triel centres-Comparison with hydrogen bond interaction. Coord. Chem. Rev. 2020, 407, 213171. [Google Scholar] [CrossRef]
- Xu, Z.F.; Li, Y. Triel bonds in RZH(2)center dot center dot center dot NH3: Hybridization, solvation, and substitution. J. Mol. Model. 2019, 25, 219. [Google Scholar] [CrossRef]
- Jablonski, M. Hydride-Triel Bonds. J. Comput. Chem. 2018, 39, 1177–1191. [Google Scholar] [CrossRef]
- Grabowski, S.J. Two faces of triel bonds in boron trihalide complexes. J. Comput. Chem. 2018, 39, 472–480. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Mousavian, P. The triel bond: A potential force for tuning anion-pi interactions. Mol. Phys. 2018, 116, 388–398. [Google Scholar] [CrossRef]
- Grabowski, S.J. Triel bonds-complexes of boron and aluminum trihalides and trihydrides with benzene. Struct. Chem. 2017, 28, 1163–1171. [Google Scholar] [CrossRef]
- Grabowski, S.J. pi-Hole Bonds: Boron and Aluminum Lewis Acid Centers. Chemphyschem 2015, 16, 1470–1479. [Google Scholar] [CrossRef] [PubMed]
- Burgi, H.B.; Dunitz, J.D.; Shefter, E. Geometrical reaction coordinates. II. Nucleophilic addition to a carbonyl group. J. Am. Chem. Soc. 1973, 95, 5065–5067. [Google Scholar] [CrossRef]
- Harder, M.; Kuhn, B.; Diederich, F. Efficient Stacking on Protein Amide Fragments. Chemmedchem 2013, 8, 397–404. [Google Scholar] [CrossRef]
- Bartlett, G.J.; Choudhary, A.; Raines, R.T.; Woolfson, D.N. n ->pi* interactions in proteins. Nat. Chem. Biol. 2010, 6, 615–620. [Google Scholar] [CrossRef]
- Andleeb, H.; Khan, I.; Bauza, A.; Tahir, M.N.; Simpson, J.; Hameed, S.; Frontera, A. Synthesis and supramolecular self-assembly of thioxothiazolidinone derivatives driven by pi-bonding and diverse 7 hole interactions: A combined experimental and theoretical analysis. J. Mol. Struct. 2017, 1139, 209–221. [Google Scholar] [CrossRef]
- Gomila, R.M.; Frontera, A. Covalent and Non-covalent Noble Gas Bonding Interactions in XeFn Derivatives (n = 2–6): A Combined Theoretical and ICSD Analysis. Front. Chem. 2020, 8, 395. [Google Scholar] [CrossRef]
- Bauza, A.; Frontera, A. sigma/pi-Hole noble gas bonding interactions: Insights from theory and experiment. Coord. Chem. Rev. 2019, 404, 213112. [Google Scholar] [CrossRef]
- Esrafili, M.D.; Vessally, E. The strengthening effect of a hydrogen or lithium bond on the Z···N aerogen bond (Z = Ar, Kr and Xe): A comparative study. Mol. Phys. 2016, 114, 3265–3276. [Google Scholar] [CrossRef]
- Bauza, A.; Frontera, A. pi-Hole aerogen bonding interactions. Phys. Chem. Chem. Phys. 2015, 17, 24748–24753. [Google Scholar] [CrossRef] [PubMed]
- Bauzá, A.; Frontera, A. Aerogen Bonding Interaction: A New Supramolecular Force? Angew. Chem. Int. Ed. 2015, 54, 7340–7343. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Frontera, A. Not Only Hydrogen Bonds: Other Noncovalent Interactions. Crystals 2020, 10, 180. [Google Scholar] [CrossRef] [Green Version]
- Hou, M.C.; Zhu, Y.F.; Li, Q.Z.; Scheiner, S. Tuning the Competition between Hydrogen and Tetrel Bonds by a Magnesium Bond. Chemphyschem 2020, 21, 212–219. [Google Scholar] [CrossRef]
- Alkorta, I.; Legon, A.C. Non-Covalent Interactions Involving Alkaline-Earth Atoms and Lewis Bases B: An ab Initio Investigation of Beryllium and Magnesium Bonds, B center dot center dot center dot MR2 (M = Be or Mg, and R = H, F or CH3). Inorganics 2019, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, S.J. Magnesium Bonds: From Divalent Mg Centres to Trigonal and Tetrahedral Coordination. Chemistryselect 2018, 3, 3147–3154. [Google Scholar] [CrossRef]
- Alikhani, M.E. Beryllium bonding: Insights from the sigma- and pi-hole analysis. J. Mol. Model. 2020, 26, 94. [Google Scholar] [CrossRef]
- Zhang, Z.; Lu, T.; Ding, L.Y.; Wang, G.Y.; Wang, Z.X.; Zheng, B.S.; Liu, Y.; Ding, X.L. Cooperativity effects between regium-bonding and pnicogen-bonding interactions in ternary MF center dot center dot center dot PH3O center dot center dot center dot MF (M = Cu, Ag, Au): An ab initio study. Mol. Phys. 2020, 118, e1784478. [Google Scholar] [CrossRef]
- Wang, R.J.; Wang, Z.; Yu, X.F.; Li, Q.Z. Synergistic and Diminutive Effects between Regium and Aerogen Bonds. Chemphyschem 2020, 21, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Rivalry between Regium and Hydrogen Bonds Established within Diatomic Coinage Molecules and Lewis Acids/Bases. Chemphyschem 2020, 21, 2557–2563. [Google Scholar] [CrossRef]
- Alkorta, I.; Trujillo, C.; Sanchez-Sanz, G.; Elguero, J. Regium Bonds between Silver(I) Pyrazolates Dinuclear Complexes and Lewis Bases (N-2, OH2, NCH, SH2, NH3, PH3, CO and CNH). Crystals 2020, 10, 137. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Understanding Regium Bonds and their Competition with Hydrogen Bonds in Au-2:HX Complexes. Chemphyschem 2019, 20, 1572–1580. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Regium bonds between Mn clusters (M = Cu, Ag, Au and n = 2–6) and nucleophiles NH3 and HCN. Phys. Chem. Chem. Phys. 2018, 20, 22498–22509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, T.; Li, D.; Cheng, L.J. Theoretical analysis of the spodium bonds in HgCl2 center dot center dot center dot L (L = ClR, SR2, and PR3) dimers. Chem. Phys. 2020, 539, 110978. [Google Scholar] [CrossRef]
- Mahmoudi, G.; Masoudiasl, A.; Babashkina, M.G.; Frontera, A.; Doert, T.; White, J.M.; Zangrando, E.; Zubkov, F.I.; Safin, D.A. On the importance of pi-hole spodium bonding in tricoordinated Hg-II complexes. Dalton T 2020, 49, 17547–17551. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, G.; Lawrence, S.E.; Cisterna, J.; Cardenas, A.; Brito, I.; Frontera, A.; Safin, D.A. A new spodium bond driven coordination polymer constructed from mercury(ii) azide and 1,2-bis(pyridin-2-ylmethylene)hydrazine. New J. Chem. 2020, 44, 21100–21107. [Google Scholar] [CrossRef]
- Bauze, A.; Alkorta, I.; Elguero, J.; Mooibroek, T.J.; Frontera, A. Spodium Bonds: Noncovalent Interactions Involving Group 12 Elements. Angew. Chem. Int. Ed. 2020, 59, 17482–17487. [Google Scholar] [CrossRef]
- Frontera, A. σ- and π-Hole Interactions. Crystals 2020, 10, 721. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The halogen bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esrafili, M.D.; Mohammadirad, N. An ab initio study on tunability of σ-hole interactions in XHS:PH2Y and XH2P:SHY complexes (X = F, Cl, Br; Y = H, OH, OCH3, CH3, C2H5, and NH2). J. Mol. Model. 2015, 21, 2727. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability II: The varying roles of electrostatic and dispersion contributions to attraction in halogen bonds. J. Mol. Model. 2013, 19, 4651–4659. [Google Scholar] [CrossRef]
- Riley, K.E.; Murray, J.S.; Fanfrlik, J.; Rezac, J.; Sola, R.J.; Concha, M.C.; Ramos, F.M.; Politzer, P. Halogen bond tunability I: The effects of aromatic fluorine substitution on the strengths of halogen-bonding interactions involving chlorine, bromine, and iodine. J. Mol. Model. 2011, 17, 3309–3318. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. sigma-Hole Interactions: Perspectives and Misconceptions. Crystals 2017, 7, 212. [Google Scholar] [CrossRef] [Green Version]
- Bulat, F.A.; Toro-Labbe, A.; Brinck, T.; Murray, J.S.; Politzer, P. Quantitative analysis of molecular surfaces: Areas, volumes, electrostatic potentials and average local ionization energies. J. Mol. Model. 2010, 16, 1679–1691. [Google Scholar] [CrossRef]
- Murray, J.S.; Politzer, P. Molecular electrostatic potentials and noncovalent interactions. Wires Comput. Mol. Sci. 2017, 7, e13260. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. sigma-holes and pi-holes: Similarities and differences. J. Comput. Chem. 2018, 39, 464–471. [Google Scholar] [CrossRef]
- Clark, T.; Murray, J.S.; Politzer, P. Role of Polarization in Halogen Bonds. Aust. J. Chem. 2014, 67, 451–456. [Google Scholar] [CrossRef] [Green Version]
- Hennemann, M.; Murray, J.S.; Politzer, P.; Riley, K.E.; Clark, T. Polarization-induced sigma-holes and hydrogen bonding. J. Mol. Model. 2012, 18, 2461–2469. [Google Scholar] [CrossRef] [PubMed]
- Riley, K.E.; Hobza, P. The relative roles of electrostatics and dispersion in the stabilization of halogen bonds. Phys. Chem. Chem. Phys. 2013, 15, 17742–17751. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Aerogen bonds formed between AeOF(2) (Ae = Kr, Xe) and diazines: Comparisons between sigma-hole and pi-hole complexes. Phys. Chem. Chem. Phys. 2018, 20, 4676–4687. [Google Scholar] [CrossRef]
- Zierkiewicz, W.; Michalczyk, M.; Wysokinski, R.; Scheiner, S. On the ability of pnicogen atoms to engage in both sigma and pi-hole complexes. Heterodimers of ZF2C6H5 (Z = P, As, Sb, Bi) and NH3. J. Mol. Model. 2019, 25, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wysokinski, R.; Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. How Many Pnicogen Bonds can be Formed to a Central Atom Simultaneously? J. Phys. Chem. A 2020, 124, 2046–2056. [Google Scholar] [CrossRef] [PubMed]
- Zierkiewicz, W.; Wysokinski, R.; Michalczyk, M.; Scheiner, S. Chalcogen bonding of two ligands to hypervalent YF4 (Y = S, Se, Te, Po). Phys. Chem. Chem. Phys. 2019, 21, 20829–20839. [Google Scholar] [CrossRef] [PubMed]
- Michalczyk, M.; Zierkiewicz, W.; Wysokinski, R.; Scheiner, S. Hexacoordinated Tetrel-Bonded Complexes between TF4 (T =Si, Ge, Sn, Pb) and NCH: Competition between sigma- and pi-Holes. Chemphyschem 2019, 20, 959–966. [Google Scholar] [CrossRef] [Green Version]
- Makarewicz, E.; Lundell, J.; Gordon, A.J.; Berski, S. On the nature of interactions in the F2 OXe(...) NCCH3 complex: Is there the Xe(IV)N bond? J. Comput. Chem. 2016, 37, 1876–1886. [Google Scholar] [CrossRef]
- Brock, D.S.; Bilir, V.; Mercier, H.P.A.; Schrobilgen, G.J. XeOF2, F2OXeN equivalent to CCH3, and XeOF2 center dot nHF: Rare examples of Xe(IV) oxide fluorides. J. Am. Chem. Soc. 2007, 129, 3598–3611. [Google Scholar] [CrossRef]
- Brock, D.S.; de Pury, J.J.C.; Mercier, H.P.A.; Schrobilgen, G.J.; Silvi, B. A Rare Example of a Krypton Difluoride Coordination Compound: [BrOF2][AsF6]center dot 2KrF(2). J. Am. Chem. Soc. 2010, 132, 3533–3542. [Google Scholar] [CrossRef]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, Y.-L.; Yang, J.; Zhou, P.-P.; Xie, K. Noncovalent functionalization of graphene via π-hole···π and σ-hole···π interactions. Struct. Chem. 2020, 31, 97–101. [Google Scholar] [CrossRef]
- Yang, F.-L.; Yang, X.; Wu, R.-Z.; Yan, C.-X.; Yang, F.; Ye, W.; Zhang, L.-W.; Zhou, P.-P. Intermolecular interactions between σ- and π-holes of bromopentafluorobenzene and pyridine: Computational and experimental investigations. Phys. Chem. Chem. Phys. 2018, 20, 11386–11395. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.F.; Chen, X.; Wu, W.X.; Zhang, G.Q.; Li, X.; Li, Z.Z.; Jin, W.J. 1,3,5-Trifluoro-2,4,6-triiodobenzene: A neglected NIR phosphor with prolonged lifetime by σ-hole and π-hole capture. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 224, 117428. [Google Scholar] [CrossRef]
- Shukla, R.; Khan, I.; Ibrar, A.; Simpson, J.; Chopra, D. Complex electronic interplay of σ-hole and π-hole interactions in crystals of halogen substituted 1,3,4-oxadiazol-2(3H)-thiones. Crystengcomm 2017, 19, 3485–3498. [Google Scholar] [CrossRef]
- Li, L.; Wang, H.; Wang, W.; Jin, W.J. Interactions between haloperfluorobenzenes and fluoranthene in luminescent cocrystals from π-hole⋯π to σ-hole⋯π bonds. Crystengcomm 2017, 19, 5058–5067. [Google Scholar] [CrossRef]
- Wang, J.; Mo, L.; Li, X.; Geng, Z.; Zeng, Y. The protonated 2-halogenated imidazolium cation as the noncovalent interaction donor: The σ-hole and π-hole interactions. J. Mol. Model. 2016, 22, 299. [Google Scholar] [CrossRef]
- Zhang, Y.; Ji, B.M.; Tian, A.M.; Wang, W.Z. Communication: Competition between pi center dot center dot center dot pi interaction and halogen bond in solution: A combined C-13 NMR and density functional theory study. J. Chem. Phys. 2012, 136, 141101. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Zhang, Y.; Ji, B.M.; Tian, A.M.; Wang, W.Z. Structural Competition between Halogen Bonds and Lone-Pair center dot center dot center dot p Interactions in Solution. Chemphyschem 2012, 13, 1411–1414. [Google Scholar] [CrossRef] [PubMed]
- Shukla, R.; Claiser, N.; Souhassou, M.; Lecomte, C.; Balkrishna, S.J.; Kumar, S.; Chopra, D. Exploring the simultaneous [sigma]-hole/[pi]-hole bonding characteristics of a Br...[pi] interaction in an ebselen derivative via experimental and theoretical electron-density analysis. Iucrj 2018, 5, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Lang, T.; Li, X.; Meng, L.; Zheng, S.; Zeng, Y. The cooperativity between the σ-hole and π-hole interactions in the ClO···XONO2/XONO···NH3 (X = Cl, Br, I) complexes. Struct. Chem. 2015, 26, 213–221. [Google Scholar] [CrossRef]
- Solimannejad, M.; Nassirinia, N.; Amani, S. A computational study of 1:1 and 1:2 complexes of nitryl halides (O2NX) with HCN and HNC. Struct. Chem. 2013, 24, 651–659. [Google Scholar] [CrossRef]
- Solimannejad, M.; Ramezani, V.; Trujillo, C.; Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. Competition and Interplay between sigma-Hole and pi-Hole Interactions: A Computational Study of 1:1 and 1:2 Complexes of Nitryl Halides (O2NX) with Ammonia. J. Phys. Chem. A 2012, 116, 5199–5206. [Google Scholar] [CrossRef] [PubMed]
- McDowell, S.A.C.; Joseph, J.A. A comparative study of model halogen-bonded, π-hole-bonded and cationic complexes involving NCX AND H2O (X = F, Cl, Br). Mol. Phys. 2015, 113, 16–21. [Google Scholar] [CrossRef]
- Bauza, A.; Frontera, A. On the Versatility of BH2X (X = F, Cl, Br, and I) Compounds as Halogen-, Hydrogen-, and Triel-Bond Donors: An AbInitio Study. Chemphyschem 2016, 17, 3181–3186. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Nagendra, G.; Samarasimhareddy, M.; Sureshbabu, V.V.; Guru Row, T.N. Observation of a reversible isomorphous phase transition and an interplay of “σ-holes” and “π-holes” in Fmoc-Leu-ψ[CH2-NCS]. Chem. Commun. 2015, 51, 933–936. [Google Scholar] [CrossRef]
- Dong, W.; Wang, Y.; Cheng, J.; Yang, X.; Li, Q. Competition between σ-hole pnicogen bond and π-hole tetrel bond in complexes of CF2=CFZH2 (Z = P, As, and Sb). Mol. Phys. 2019, 117, 251–259. [Google Scholar] [CrossRef]
- Dong, W.; Yang, X.; Cheng, J.; Li, W.; Li, Q. Comparison for σ-hole and π-hole tetrel-bonded complexes involving F2CCFTF3 (TC, Si, and Ge): Substitution, hybridization, and solvation effects. J. Fluor. Chem. 2018, 207, 38–44. [Google Scholar] [CrossRef]









| Molecule | Hole Source | Hole Type | VS,max | References |
|---|---|---|---|---|
| Aerogen Bond Donors | ||||
| KrOF2 | Kr | σ | 58.7 | [132] |
| KrOF2 | Kr | π | 39.1 | [132] |
| XeOF2 | Xe | σ | 63.4 | [132] |
| XeOF2 | Xe | σ | 90 | [104] |
| XeOF2 | Xe | π | 36.2 | [132] |
| Pnicogen Bond Donors | ||||
| PF2C6H5 | P | σ | 19.4 | [133] |
| PF2C6H5 | P | π | 36.2 | * |
| AsF2C6H5 | As | σ | 28.2 | [133] |
| AsF2C6H5 | As | π | 44.0 | * |
| SbF2C6H5 | Sb | σ | 38.4 | [133] |
| SbF2C6H5 | Sb | π | 56.6 | * |
| BiF2C6H5 | Bi | σ | 52.6 | [133] |
| BiF2C6H5 | Bi | π | 60.9 | * |
| PF3 | P | σ | 35.6 | [134] |
| PF3 | P | π | 9.7 | [134] |
| AsF3 | As | σ | 43.9 | [134] |
| AsF3 | As | π | 7.1 | [134] |
| SbF3 | Sb | σ | 51.6 | [134] |
| SbF3 | Sb | π | 10.6 | [134] |
| BiF3 | Bi | σ | 61.5 | [134] |
| BiF3 | Bi | π | 12.7 | [134] |
| Chalcogen Bond Donors | ||||
| SF4 | S | σ | 41.7 | [135] |
| SF4 | S | π | 64.4 | [135] ** |
| SeF4 | Se | σ | 51.2 | [135] |
| SeF4 | Se | π | 61.1 | [135] ** |
| TeF4 | Te | σ | 59.2 | [135] |
| TeF4 | Te | π | 54.5 | [135] ** |
| PoF4 | Po | σ | 76.3 | [135] |
| PoF4 | Po | π | 53.2 | [135] ** |
| Tetrel Bond Donors | ||||
| SiF4 | Si | σ | 127.3 | [136] |
| SiF4 | Si | π | 109.8 | [136] |
| GeF4 | Ge | σ | 120.9 | [136] |
| GeF4 | Ge | π | 106.1 | [136] |
| SnF4 | Sn | σ | 129.4 | [136] |
| SnF4 | Sn | π | 121.3 | [136] |
| PbF4 | Pb | σ | 127.3 | [136] |
| PbF4 | Pb | π | 104.1 | [136] |
| Molecule | Hole Source | Hole Type | VS,max | References |
|---|---|---|---|---|
| Halogen-Pnicogen | ||||
| XONO (X = F, Cl, Br, I) | X | σ | −6.3 to 51.1 | [150] |
| XONO (X = F, Cl, Br, I) | N | π | 20.8 to 29.5 | [150] |
| XONO2 (X = F, Cl, Br, I) | X | σ | 2.7 to 67.7 | [150] |
| XONO2 (X = F, Cl, Br, I) | N | π | 29.6 to 41.2 | [150] |
| NO2X (X = Cl, Br) | X | σ | 13.2 and 19.0 | [151,152] |
| NO2X (X = Cl, Br) | N | π | 28.1 and 29.8 | [151,152] |
| NO2I | I | σ | 29.4 | [152] |
| NO2I | N | π | 23.5 | [152] |
| Halogen-Tetrel | ||||
| NCX (X = F, Cl, Br) | X | σ | 14.3 to 42.1 | [153] |
| NCX (X = F, Cl, Br) | C | π | 12.4 to 27.3 | [153] |
| Halogen-Triel | ||||
| BH2X (X = F, Cl, Br, I) | Cl, Br, I | σ | 3.5 to 11.3 | [154] |
| BH2X (X = F, Cl, Br, I) | B | π | 28.8 to 39.4 | [154] |
| Halogen-Beryllium | ||||
| BeCl2 | Cl | σ | 1.2 | [109] |
| BeCl2 | Be | π | 32.2 | [109] |
| Molecule | Hole Source | Hole Type | VS,max | References |
|---|---|---|---|---|
| Chalcogen-Tetrel/Pnicogen | ||||
| Fmoc-Leu-Ψ[CH2NCS] | S | σ | 3.9 (exp.), 7.8 (theory) | [155] |
| Fmoc-Leu-Ψ[CH2NCS] | C=N bond | π | no data | [155] |
| Pnicogen-Tetrel | ||||
| CF2=CFZH2 (Z = P, As, Sb) | Z | σ | 19.4 to 28.9 | [156] |
| CF2=CFPF2 | C | π | 36.4 | [156] |
| CF2=CFZH2 (Z = P, As, Sb) | Z | σ | 25.7 to 28.2 | [156] |
| CF2=CFPF2 | C | π | 40.1 | [156] |
| Tetrel-Tetrel | ||||
| F2C=CFTF3 (T = C, Si, Ge) | T | σ | 8.2 to 46.4 * | [157] |
| F2C=CFTF3 (T = C, Si, Ge) | C=C Bond | π | 30.7 to 35.1 * | [157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zierkiewicz, W.; Michalczyk, M.; Scheiner, S. Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons. Molecules 2021, 26, 1740. https://doi.org/10.3390/molecules26061740
Zierkiewicz W, Michalczyk M, Scheiner S. Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons. Molecules. 2021; 26(6):1740. https://doi.org/10.3390/molecules26061740
Chicago/Turabian StyleZierkiewicz, Wiktor, Mariusz Michalczyk, and Steve Scheiner. 2021. "Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons" Molecules 26, no. 6: 1740. https://doi.org/10.3390/molecules26061740
APA StyleZierkiewicz, W., Michalczyk, M., & Scheiner, S. (2021). Noncovalent Bonds through Sigma and Pi-Hole Located on the Same Molecule. Guiding Principles and Comparisons. Molecules, 26(6), 1740. https://doi.org/10.3390/molecules26061740