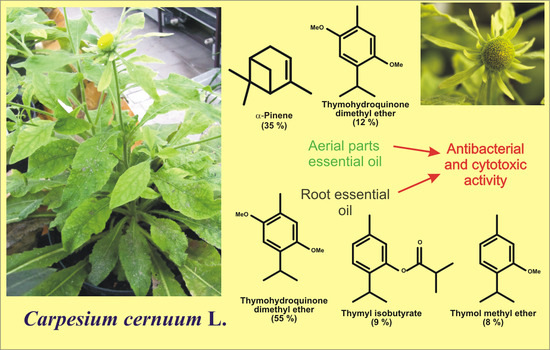
Composition of Essential Oils from Roots and Aerial Parts of Carpesium cernuum and Their Antibacterial and Cytotoxic Activities
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Plant Material
4.3. Isolation of Essential Oil
4.4. Isolation and NMR Analysis of Major Volatile Components
4.5. Identification of Essential Oil Constituents
4.6. Antibacterial Activity of Carpesium Cernuum Essential Oils
4.6.1. Bacterial Lines and Culture Conditions
4.6.2. Determination of the Minimum Inhibitory Concentrations (MICs)
4.7. Cytotoxic Activity of Essential Oils from Carpesium Cernuum
4.7.1. Cell Lines and Culture Conditions
4.7.2. Cell-Viability Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Zhang, J.P.; Wang, G.W.; Tian, X.H.; Yang, Y.X.; Liu, Q.X.; Chen, L.P.; Li, H.L.; Zhang, W.D. The genus Carpesium: A review of its ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharm. 2015, 163, 173–191. [Google Scholar] [CrossRef]
- Geng, Y.; Zhang, Y.; Ranjitkar, S.; Huai, H.; Wang, Y. Traditional knowledge and its transmission of wild edibles used by the Naxi in Baidi Village, northwest Yunnan province. J. Ethnobiol. Ethnomed. 2016, 12, 10. [Google Scholar] [CrossRef] [Green Version]
- Hong, L.; Zhuo, J.; Lei, Q.; Zhou, J.; Ahmed, S.; Wang, C.; Long, Y.; Li, F.; Long, C. Ethnobotany of wild plants used for starting fermented beverages in Shui communities of southwest China. J. Ethnobiol. Ethnomed. 2015, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- EFloras. 2008. Available online: http://www.efloras.org (accessed on 14 February 2021). Missouri Botanical Garden, St. Louis, MO & Harvard University Herbaria, Cambridge, MA.
- Xu, Z.; Chang, L. Asteraceae. In Identification and Control of Common Weeds: Volume 3, 1st ed.; Zhejiang University Press: Hangzhou, China; Springer Nature: Singapore, 2017; pp. 441–721. [Google Scholar]
- POWO. Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. 2019. Available online: http://www.plantsoftheworldonline.org (accessed on 15 February 2021).
- Greuter, W. (2006+): Compositae (pro Parte Majore)–In: Greuter, W. & Raab-Straube, E. von (ed.): Compositae. Euro + Med Plant-base—the Information Resource for Euro-Mediterranean Plant Diversity. Available online: http://ww2.bgbm.org/EuroPlusMed/ (accessed on 15 February 2021).
- Park, Y.J.; Cheon, S.Y.; Lee, D.S.; Cominguez, D.C.; Zhang, Z.; Lee, S.; An, H.J. Anti-inflammatory and antioxidant effects of Carpesium cernuum L. methanolic extract in LPS-stimulated RAW 264.7 macrophages. Mediat. Inflamm. 2020, 2020, 3164239. [Google Scholar] [CrossRef] [PubMed]
- Dang, H.; Li, H.; Ma, C.; Wang, Y.; Tian, J.; Deng, L.; Wang, D.; Jing, X.; Luo, K.; Xing, W.; et al. Identification of Carpesium cernuum extract as a tumor migration inhibitor based on its biological response profiling in breast cancer cells. Phytomedicine 2019, 64, 153072. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Larruscain, D.; Santos-Vicente, M.; Anderberg, A.A.; Rico, E.; Martínez-Ortega, M.M. Phylogeny of the Inula group (Asteraceae: Inuleae): Evidence from nuclear and plastid genomes and a recircumscription of Pentanema. Taxon 2018, 67, 149–164. [Google Scholar] [CrossRef]
- Liu, L.L.; Wang, R.; Yang, J.L.; Shi, Y.P. Diversity of sesquiterpenoids from Carpesium cernuum. Helv. Chim. Acta 2010, 93, 595–601. [Google Scholar] [CrossRef]
- Liu, Q.X.; Yang, Y.X.; Zhang, J.P.; Chen, L.P.; Shen, Y.H.; Li, H.L.; Zhang, W.D. Isolation, structure elucidation, and absolute configuration of highly oxygenated germacranolides from Carpesium cernuum. J. Nat. Prod. 2016, 79, 2472–2478. [Google Scholar] [CrossRef]
- Yan, C.; Zhang, W.Q.; Sun, M.; Liang, W.; Wang, T.Y.; Zhang, Y.D.; Ding, X. Carpescernolides A and B, rare oxygen bridge-containing sesquiterpene lactones from Carpesium cernuum. Tetrahedron Lett. 2018, 59, 4063–4066. [Google Scholar] [CrossRef]
- Wang, Q.; Pan, L.; Lin, L.; Zhang, R.; Du, Y.; Chen, H.; Huang, M.; Guo, K.; Yang, X. Essential oil from Carpesium abrotanoides L. induces apoptosis via activating mitochondrial pathway in hepatocellular carcinoma cells. Curr. Med. Sci. 2018, 38, 1045–1055. [Google Scholar] [CrossRef]
- Kameoka, H.; Sagara, K.; Miyazawa, M. Components of essential oils of Kakushitsu (Daucus carota L. and Carpesium abrotanoides L.). Nippon Nōgeikagaku Kaishi 1989, 63, 185–188. [Google Scholar] [CrossRef]
- Wajs-Bonikowska, A.; Malarz, J.; Stojakowska, A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium divaricatum, a Traditional Herbal Medicine and Wild Edible Plant from South-East Asia, Grown in Poland. Molecules 2019, 24, 4418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchese, A.; Orhan, I.E.; Daglia, M.; Barbieri, R.; Di Lorenzo, A.; Nabavi, S.F.; Gortzi, O.; Izadi, M.; Nabavi, S.M. Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem. 2016, 210, 402–414. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]
- Anderberg, A.A. Inuleae. In Systematics, Evolution and Biogeography of Compositae, 1st ed.; Funk, V.A., Susanna, A., Stuessy, T.F., Bayer, R.J., Eds.; International Association for Plant Taxonomy: Vienna, Austria, 2009; pp. 667–680. [Google Scholar]
- Wajs-Bonikowska, A.; Stojakowska, A.; Kalemba, D. Chemical composition of essential oils from a multiple shoot culture of Telekia speciosa and different plant organs. Nat. Prod. Commun. 2012, 7, 625–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owolabi, M.S.; Lajide, L.; Villanueva, H.E.; Setzer, W.N. Essential oil composition and insecticidal activity of Blumea perrottetiana growing in Southwestern Nigeria. Nat. Prod. Commun. 2010, 5, 1135–1138. [Google Scholar] [PubMed] [Green Version]
- Joshi, R.K. Chemical composition of Blumea virens roots from India. Chem. Nat. Compd. 2018, 54, 584–585. [Google Scholar] [CrossRef]
- Xu, T.; Gherib, M.; Bekhechi, C.; Atik-Bekkara, F.; Casabianca, H.; Tomi, F.; Casanova, J.; Bighelli, A. Thymyl esters derivatives and a new natural product modhephanone from Pulicaria mauritanica Coss. (Asteraceae) root oil. Flavour Fragr. J. 2015, 30, 83–90. [Google Scholar] [CrossRef]
- Onayade, O.A.; Scheffer, J.J.C.; Schripsema, J.; van der Gen, A. 6-Hydroxycarvotanacetone and other constituents of the essential oil of Laggera alata (D. Don) Sch. Bip. ex Oliv. Flavour Fragr. J. 1990, 5, 165–172. [Google Scholar] [CrossRef]
- Verma, R.S.; Padalia, R.C.; Chauhan, A. Compositional variation in the essential oils of vegetative and reproductive parts of Laggera crispata (Vahl) Hepper & Wood. Natl. Acad. Sci. Lett. 2013, 36, 447–451. [Google Scholar]
- Verma, R.S.; Padalia, R.C.; Chanotiya, C.S.; Chauhan, A.; Yadav, A. Chemical investigation of the essential oil of Laggera crispata (Vahl) Hepper & Wood from India. J. Serb. Chem. Soc. 2011, 76, 523–528. [Google Scholar]
- Kambiré, D.A.; Boti, J.B.; Yapi, T.A.; Ouattara, Z.A.; Paoli, M.; Bighelli, A.; Tomi, F.; Casanova, J. Composition and Intraspecific chemical variability of leaf essential oil of Laggera pterodonta from Côte d’Ivoire. Chem. Biodiv. 2020, 17, e1900504. [Google Scholar] [CrossRef] [Green Version]
- Kambiré, D.A.; Yapi, A.T.; Boti, J.B.; Ouattara, Z.A.; Tonzibo, Z.F.; Filippi, J.-J. Two new eudesman-4α-ol epoxides from the stem essential oil of Laggera pterodonta from Côte d’Ivoire. Nat. Prod. Res. 2020, 34, 2765–2771. [Google Scholar] [CrossRef] [PubMed]
- Getahun, T.; Sharma, V.; Kumar, D.; Gupta, N. Chemical composition, and antibacterial and antioxidant activities of the essential oils from Laggera tomentosa Sch. Bip. ex Oliv. et Hiern (Asteraceae). Turk. J. Chem. 2020, 44, 1539–1548. [Google Scholar] [CrossRef]
- Jirovetz, L.; Buchbauer, G.; Shahabi, M.; Shafi, P.M.; Rajeeve, K.R. Medicinal used plants from India: Analysis of the essential oils of Sphaeranthus indicus flowers, roots and stems with leaves. Sci. Pharm. 2003, 71, 251–259. [Google Scholar] [CrossRef] [Green Version]
- Duñg, N.X.; Târn, N.T.; Kruk, C.; Leclercq, P.A. Composition of the oil of Eupatorium stoechadosmum Hance from Vietnam. J. Essent. Oil Res. 1991, 3, 115–116. [Google Scholar] [CrossRef]
- Tabanca, N.; Bernier, U.R.; Tsikolia, M.; Becnel, J.J.; Sampson, B.; Werle, C.; Demirci, B.; Başer, K.H.C.; Blythe, E.K.; Pounders, C.; et al. Eupatorium capillifolium essential oil: Chemical composition, antifungal activity, and insecticidal activity. Nat. Prod. Commun. 2010, 5, 1409–1415. [Google Scholar] [CrossRef]
- Solis-Quispe, L.; Pino, J.A.; Falco, A.S.; Tomaylla-Cruz, C.; Quispe-Tonccochi, E.G.; Solis-Quispe, J.A. Chemical composition and antibacterial activities of essential oil from Ageratina pentlandiana (DC.) R.M. King & H. Rob. leaves grown in the Peruvian Andes. J. Essent. Oil Res. 2019, 31, 409–413. [Google Scholar]
- Mala, J.G.S.; Zoghbi, M.G.B.; da Silva, M.H.L.; Andrade, E.H. A Essential oils of Eupatorium triplinerve Vahl and E. paniculatum Poepp. et Endl. J. Essent. Oil Res. 1999, 11, 541–544. [Google Scholar] [CrossRef]
- Gauvin-Bialecki, A.; Marodon, C. Essential oil of Ayapana triplinervis from Reunion Island: A good natural source of thymohydroquinone dimethyl ether. Biochem. Syst. Ecol. 2009, 36, 853–858. [Google Scholar] [CrossRef]
- Haddad, J.G.; Picard, M.; Bénard, S.; Desvignes, C.; Desprès, P.; Diotel, N.; El Kalamouni, C. Ayapana triplinervis essential oil and its main component thymohydroquinone dimethyl ether inhibit Zika virus at doses devoid of toxicity in zebrafish. Molecules 2019, 24, 3447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pljevljakušić, D.; Rančić, D.; Ristić, M.; Vujisić, L.; Radanović, D.; Dajić-Stevanović, Z. Rhizome and root yield of the cultivated Arnica montana L., chemical composition and histochemical localization of essential oil. Ind. Crops Prod. 2012, 39, 177–189. [Google Scholar] [CrossRef]
- Sugier, D.; Sugier, P.; Jakubowicz-Gil, J.; Winiarczyk, K.; Kowalski, R. Essential oil from Arnica montana achenes: Chemical characteristics and anticancer activity. Molecules 2019, 24, 4158. [Google Scholar] [CrossRef] [Green Version]
- Sugier, P.; Jakubowicz-Gil, J.; Sugier, D.; Kowalski, R.; Gawlik-Dziki, U.; Kołodziej, B.; Dziki, D. Chemical characteristics and anticancer activity of essential oil from Arnica montana L. rhizomes and roots. Molecules 2020, 25, 1284. [Google Scholar] [CrossRef] [Green Version]
- Joshi, R.K. Chemical constituents and antibacterial property of the essential oil of the roots of Cyathocline purpurea. J. Ethnopharm. 2013, 145, 621–625. [Google Scholar] [CrossRef]
- Pande, C.; Tewari, G.; Singh, C.; Singh, S. Essential oil composition of aerial parts of Cyclospermum leptophyllum (Pers.) Sprague ex Britton and P. Wilson. Nat. Prod. Res. 2011, 25, 592–595. [Google Scholar] [CrossRef]
- Getahun, T.; Sharma, V.; Gupta, N. Chemical composition and biological activity of essential oils from Aloe debrana roots. J. Essent. Oil Bear. Pl. 2020, 23, 493–502. [Google Scholar] [CrossRef]
- Kwiatkowski, P.; Łopusiewicz, Ł.; Pruss, A.; Kostek, M.; Sienkiewicz, M.; Bonikowski, R.; Wojciechowska-Koszko, I.; Dołęgowska, B. Antibacterial activity of selected essential oil compounds alone and in combination with β-lactam antibiotics against MRSA strains. Int. J. Mol. Sci. 2020, 21, 7106. [Google Scholar] [CrossRef]
- Satooka, H.; Kubo, I. Effects of thymol on B16-F10 melanoma cells. J. Agric. Food Chem. 2012, 60, 2746–2752. [Google Scholar] [CrossRef]
- Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria that Grow Aerobically, Approved Standard, 9th ed.; CLSI document M07-A9; Clinical and Laboratory Standards Institute (CLSI): Wayne, PA, USA, 2012.
- Sargent, J.M.; Taylor, C.G. Appraisal of the MTT assay as a rapid test of chemosensitivity in acute myeloid leukaemia. Br. J. Cancer 1989, 60, 206–210. [Google Scholar] [CrossRef]


| No | Compound | Aerial Arts | Roots | RI 1 | RI 2 |
|---|---|---|---|---|---|
| Amount (%) | |||||
| 1 | n-Hexanal | 0.2 | 775 | 769 | |
| 2 | (E)-2-Hexenal | 1.7 | 826 | 822 | |
| 3 | α-Pinene | 34.7 | 0.1 | 932 | 932 |
| 4 | Camphene | 0.1 | 940 | 943 | |
| 5 | Thuja-2,4(10)-diene | 0.3 | 944 | 957 | |
| 6 | Octane-2,3-dione | tr | 962 | 967 | |
| 7 | 6-Methylhept-5-en-2-one | 0.5 | 963 | 972 | |
| 8 | 1-Octen-3-ol | 0.9 | 964 | 972 | |
| 9 | β-Pinene | 0.6 | 966 | 972 | |
| 10 | 2-Pentylfuran | 0.7 | 977 | 977 | |
| 11 | Myrcene | 0.3 | 981 | 979 | |
| 12 | (E)-2-(2-Pentenyl)furan | 0.2 | 985 | 983 | |
| 13 | m-Cymene | 0.1 | 993 | 999 | |
| 14 | α-Terpinene | 0.2 | 1007 | 1013 | |
| 15 | p-Cymene | 0.2 | 1010 | 1015 | |
| 16 | 1,1,3-Trimethylcyclohexan-3-one | 0.2 | 1011 | 1019 | |
| 17 | 4,6-Dimethylhept-5-en-2-one | 0.3 | 1016 | 1119 | |
| 18 | Limonene | 0.4 | 1019 | 1025 | |
| 19 | (Z)-β-Ocimene | 0.1 | 1026 | 1029 | |
| 20 | 2,6-Dimethylhept-5-enal | 0.1 | 1034 | 1036 | |
| 21 | (E)-β-Ocimene | 0.1 | 1038 | 1041 | |
| 22 | γ-Terpinene | 0.3 | 1048 | 1051 | |
| 23 | trans-Linalooloxide (furanoid) | 0.2 | 1057 | 1058 | |
| 24 | Non-1-en-3-ol | 0.2 | 1068 | 1064 | |
| 25 | Camphen-6-ol | 0.1 | 1072 | 1070 | |
| 26 | cis-Linalooloxide (furanoid) | tr | 1075 | 1072 | |
| 27 | p-Cymenene | tr | 1076 | 1076 | |
| 28 | Terpinolene | 0.1 | 1078 | 1082 | |
| 29 | n-Nonanal | 0.3 | 1084 | 1083 | |
| 30 | Linalool | 4.3 | 1088 | 1086 | |
| 31 | Oct-1-en-3-yl acetate | tr | 1096 | 1096 | |
| 32 | α-Cyclocitral | 0.1 | 1097 | 1103 | |
| 33 | α-Campholenal | 0.4 | 1104 | 1105 | |
| 34 | Perillene | 0.2 | 1106 | 1109 | |
| 35 + 36 | Camphor + Unknown (MS: 123/86/119 M) | 0.2 | 1120 | 1123 | |
| 37 | trans-Pinocarveol | 0.4 | 1123 | 1126 | |
| 38 | p-Mentha-1,5-diene-8-ol | tr | 1125 | 1127 | |
| 39 | cis-Verbenol | 0.1 | 1129 | 1132 | |
| 40 | trans-Verbenol | 0.2 | 1130 | 1137 | |
| 41 | trans-Pinocamphone | 0.4 | 1138 | 1140 | |
| 42 + 43 | β-Phellandren-8-ol + 4-Ethylbenzaldehyde | 0.4 | 1148 | 1143 + 1147 | |
| 44 + 45 | α-Phellandren-8-ol + (2E)-4-(2-Methyl-1-cyclohexen-1-yl)-2-butenal | 0.4 | 5.6 | 1149 | 1150 + 1149 |
| 46 | Terpinen-4-ol | 0.5 | 1162 | 1164 | |
| 47 | Dimethylsiloxane pentamer (artifact) | 0.1 | 1169 | 1169 | |
| 48 | α-Terpineol | 1.0 | 1174 | 1176 | |
| 49 | Myrtenol | 0.1 | 1181 | 1178 | |
| 50 | n-Decanal | 2.2 | 1187 | 1180 | |
| 51 | Dihydrocarveol (Isomer 2) | tr | 1193 | 1193 | |
| 52 | β-Cyclocitral | 0.3 | 1197 | 1195 | |
| 53 | trans-Carveol | 0.1 | 1202 | 1200 | |
| 54 | Nerol | 0.1 | 1214 | 1210 | |
| 55 | Thymol methyl ether | 0.5 | 8.4 | 1216 | 1215 |
| 56 | β-apo-8-Carotenal | 0.1 | 1236 | 1236 | |
| 57 | Geraniol | 0.5 | 1238 | 1238 | |
| 58 | α-Ionene | 0.1 | 1243 | 1255 | |
| 59 | Thymol | tr | tr | 1269 | 1267 |
| 60 | Carvacrol | 0.1 | 1277 | 1278 | |
| 61 | Dihydroedulan I | 0.2 | 1280 | 1290 | |
| 62 | Dihydroedulan II | 0.4 | 1283 | 1296 | |
| 63 | Theaspirane (Isomer 1) | 0.1 | 1290 | 1299 | |
| 64 + 65 | Myrtenyl acetate + Theaspirane (Isomer 2) | 0.2 | 1305 | 1313 + 1313 | |
| 66 | γ-Pyronene | 0.1 | 1316 | 1336 | |
| 67 | cis-Edulan | tr | 1320 | 1328 | |
| 68 | Unknown (MS: 79/77/107 M178) | 0.1 | 1324 | ||
| 69 | Dehydro-ar-ionene | 0.1 | 1337 | 1336 | |
| 70 | α-Longipinene | 0.1 | tr | 1350 | 1360 |
| 71 | 3-Hydroxy-2,4,4-trimethylpentyl 2-methylpropanoate | tr | 1356 | 1381 | |
| 72 | (E)-β-Damascenone | 0.2 | 1363 | 1361 | |
| 73 | α-Longicyclene | tr | 1369 | 1382 | |
| 74 | Unknown (MS: 79/95/107/178 M204) | 0.3 | 1370 | – | |
| 75 | 1,2-Dihydro-1,4,6-trimethylnaphthalene | 0.1 | 1372 | 1373 | |
| 76 | 1,2-Dihydro-1,5,8-trimethylnaphthalene | 0.1 | 1375 | 1376 | |
| 77 | 1,4-Dimethoxy-2-tert-butylbenzene | tr | 1382 | 1398 | |
| 78 | β-Ionol | 0.1 | 1390 | 1400 | |
| 79 | 7,8-Dihydro-β-ionone | 0.2 | 0.2 | 1393 | 1413 |
| 80 | 2,5-Dimethoxy-p-cymene (Thymohydroquinone dimethyl ether) 3 | 11.6 | 54.8 | 1405 | 1399 |
| 81 | 2-tert-Butyl-1,4-dimethoxybenzene | 0.3 | 1410 | 1400 | |
| 81 + 82 | 2-tert-Butyl-1,4-dimethoxybenzene + Unknown: (MS: 105/119/77/147 M194) | 0.4 | 1417 | 1400 | |
| 83 | Unknown (MS: 123/121/179 M194) | 0.1 | 1421 | – | |
| 84 | Dihydropseudoionone | 0.2 | 1433 | 1434 | |
| 85 | trans-α-Bergamotene | tr | 0.3 | 1436 | 1434 |
| 86 | epi-β-Santalene | 0.4 | tr | 1443 | 1446 |
| 87 | β-Santalene | 1.0 | 1459 | 1458 | |
| 88 | 8,9-Didehydrothymyl isobutyrate | 3.2 | 2.8 | 1464 | 1458 |
| 89 | Thymyl isobutyrate | 0.6 | 9.0 | 1465 | 1462 |
| 90 | (E)-β-Ionone | 0.9 | 1472 | 1466 | |
| 91 | Neryl isobutyrate | 0.1 | 1.3 | 1477 | 1468 |
| 92 | α-Terpinyl isobutyrate | 0.1 | 1477 | 1498 | |
| 93 | (3Z,6E)-α-Farnesene | 0.5 | 1483 | 1480 | |
| 94 + 95 | α-Selinene + Unknown (MS: 99/121/155 M204) | 0.9 | 1492 | 1494 | |
| 96 | (3E,6E)-α-Farnesene | 0.4 | 1496 | 1498 | |
| 97 | β-Bisabolene | 0.3 | tr | 1501 | 1503 |
| 98 | cis/trans-Calamenene | 0.1 | 1521 | 1517 | |
| 99 | (E)-β-Caryophyllene oxide | tr | 1535 | 1545 | |
| 100 | (E)-Nerolidol | 3.3 | 0.2 | 1552 | 1553 |
| 101 | Neryl isovalerate | 0.6 | 0.6 | 1559 | 1565 |
| 102 | Geranyl isovalerate | 0.2 | 0.1 | 1565 | 1573 |
| 103 | Caryophyllene epoxide | 0.2 | 0.1 | 1571 | 1578 |
| 104 | Isoaromadendrene epoxide | 0.1 | tr | 1574 | 1592 |
| 105 | Widdrol | 0.1 | 1596 | 1601 | |
| 106 | Acora-2,4(15)-dien-11-ol | 0.1 | 1613 | 1616 | |
| 107 | α-Acorenol | 0.1 | 0.2 | 1621 | 1623 |
| 108 | 1-epi-Cubenol | tr | tr | 1624 | 1623 |
| 109 | β-Acorenol | 0.8 | 1632 | 1626 | |
| 110 | β-Eudesmol | 0.4 | tr | 1632 | 1643 |
| 111 | Tetradecan-13-olide | 0.2 | 1638 | 1643 | |
| 112 | 5β,7βH,10α-Eudesm-11-en-1α-ol | 0.3 | 0.2 | 1642 | – |
| 113 | cis-Eudesma-4,11-dien-8-ol | tr | 0.1 | 1656 | 1648 |
| 114 | 6-Methoxythymyl isobutyrate 3 | 3.3 | 5.2 | 1662 | 1658 |
| 115 | 6-Methoxy-8,9-didehydrothymyl isobutyrate 3 | 0.1 | 0.1 | 1676 | 1676 |
| 116 | (E,E)-Farnesol | 0.3 | 1703 | 1694 | |
| 117 | Benzyl Benzoate | tr | 1731 | 1730 | |
| 118 | Isobutyl phthalate (artifact) | 5.4 | 0.3 | 1834 | 1840 |
| 119 | Benzyl salicylate | 0.1 | 1842 | 1847 | |
| 120 | 9-Isobutyryloxythymyl isobutyrate | 0.8 | 1.0 | 1882 | 1891 |
| 121 | 10-isobutyryloxy-8,9-dehydrothymyl isobutyrate | 1.1 | 1.3 | 1886 | 1891 |
| 122 | Eudesma-5,11(13)-dien-8,12-olide | 0.1 | 0.4 | 1906 | 1891 |
| 123 | 7-Isobutyryloxythymyl isobutyrate 3 | 0.1 | 1921 | 1930 | |
| 124 | Butyl phthalate (artifact) | 0.5 | 1921 | 1909 | |
| 125 | Hexadecanoid acid | 0.6 | 1955 | 1942 | |
| 126 | 9-(2-Methylbutyryloxy)thymyl isobutyrate | 0.1 | 0.1 | 1968 | 1970 |
| 127 | 10-Isobutyryloxy-8,9-epoxythymyl isobutyrate 3 | 0.5 | 5.1 | 1991 | 2036 |
| 128 | Unknown (MS: 177/150/71 M290) | 0.4 | 2049 | – | |
| 129 | 10-(2-methylbutyryloxy)-8,9-epoxythymyl isobutyrate | 0.1 | 0.4 | 2077 | 2056 |
| 130 | trans-Phytol | 0.4 | 2101 | 2104 | |
| Sum of identified | 97.7 | 99.6 | |||
| Yield of the essential oil (%) | 0.015 4 | 0.170 5 | |||
| Bacterial Cell Line | C. Cernuum REO | C. Cernuum APEO | Thymol |
|---|---|---|---|
| Minimal Inhibitory Concentration (MIC) | |||
| (µL/mL) | (µg/mL) 1 | ||
| Staphylococcus aureus ATCC 29213 | 62.5 ± 0.0 | 15.6 ± 0.0 | 0.9 ± 0.0 |
| Escherichia coli ATCC 25922 | 125.0 ± 0.0 | 15.6 ± 0.0 | 7.5 ± 0.0 |
| Enterococcus faecalis ATCC 29212 | 125.0 ± 0.0 | 93.8 ± 44.2 | 1.9 ± 0.0 |
| Klebsiella pneumoniae ATCC 700603 | 125.0 ± 0.00 | 62.5 ± 0.0 | 15.0 ± 0.0 |
| Pseudomonas aeruginosa ATCC 27853 | 250.0 ± 0.0 | 93.8 ± 44.2 | 7.5 ± 0.0 |
| Serratia marcescens ATCC 13880 | >250 | 250.0 ± 0.0 | 30.0 ± 0.0 |
| Acinetobacter baumanii ATCC 19606 | 11.7 ± 5.5 | 11.7 ± 5.5 | 1.9 ± 0.0 |
| Cell Line | IC50 (nL/mL) | ||
|---|---|---|---|
| C. Cernuum REO | C. Cernuum APEO | Thymol 1 | |
| Fibroblasts | 83.02 ± 6.51 | 75.65 ± 4.83 | 65.62 ± 5.24 |
| HaCaT | 90.26 ± 6.62 | 82.14 ± 5.02 | 49.85 ± 4.12 |
| A375 | 71.66 ± 5.12 | 75.9 ± 5.78 | 62.55 ± 4.62 |
| C32 | 107.2 ± 6.40 | 82.32 ± 7.11 | 60.39 ± 6.45 |
| Family/Tribe-Subtribe | Species | Plant Organ | 2,5-Dimethoxy-p-cymene Content in EO | Literature |
|---|---|---|---|---|
| Compositae/Inuleae-Inulinae | Blumea perrottetiana DC. | Aerial parts | 30% | [21] |
| Blumea virens DC. | Roots | 28% | [22] | |
| Pulicaria mauritanica Coss. | Roots | 37% | [23] | |
| Compositae/Inuleae-Plucheinae | Laggera alata (D. Don) Sch. Bip. ex Oliv. | Herb | 44% | [24] |
| Laggera crispata (Vahl) Hepper &Wood | Different organs | 22–75% | [25,26] | |
| Laggera pterodonta (DC.) Sch. Bip ex Oliv. | Aerial parts | 31–79% | [27,28] | |
| Laggera tomentosa Sch. Bip. ex Oliv. et Hiern | Stem bark and roots | 57–65% | [29] | |
| Sphaeranthus indicus Kurz. | Roots and herb | 27–28% | [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wajs-Bonikowska, A.; Malarz, J.; Szoka, Ł.; Kwiatkowski, P.; Stojakowska, A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium cernuum and Their Antibacterial and Cytotoxic Activities. Molecules 2021, 26, 1883. https://doi.org/10.3390/molecules26071883
Wajs-Bonikowska A, Malarz J, Szoka Ł, Kwiatkowski P, Stojakowska A. Composition of Essential Oils from Roots and Aerial Parts of Carpesium cernuum and Their Antibacterial and Cytotoxic Activities. Molecules. 2021; 26(7):1883. https://doi.org/10.3390/molecules26071883
Chicago/Turabian StyleWajs-Bonikowska, Anna, Janusz Malarz, Łukasz Szoka, Paweł Kwiatkowski, and Anna Stojakowska. 2021. "Composition of Essential Oils from Roots and Aerial Parts of Carpesium cernuum and Their Antibacterial and Cytotoxic Activities" Molecules 26, no. 7: 1883. https://doi.org/10.3390/molecules26071883
APA StyleWajs-Bonikowska, A., Malarz, J., Szoka, Ł., Kwiatkowski, P., & Stojakowska, A. (2021). Composition of Essential Oils from Roots and Aerial Parts of Carpesium cernuum and Their Antibacterial and Cytotoxic Activities. Molecules, 26(7), 1883. https://doi.org/10.3390/molecules26071883