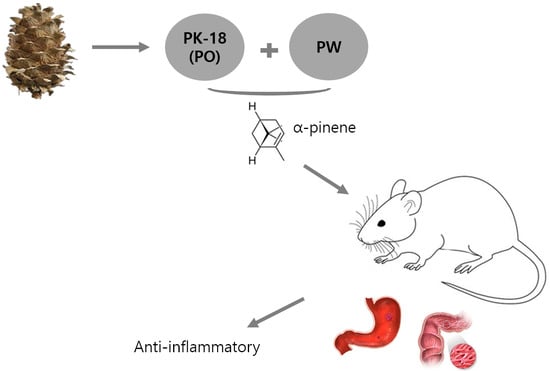
Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Phytoncide
2.1.1. Preparation of Pine Cone Extracts
2.1.2. Gas Chromatography-Mass Spectrometer Analysis of Extracts
3. Indomethacin-Induced Gastritis Rat Model
3.1. Chemicals
3.2. Animal Care
3.3. Experimental Design
3.4. Evaluation of Inflammation in Indomethacin-Induced Gastric Ulcers
3.5. Histological Evaluation
3.6. Immunohistochemistry
4. Dextran Sulfate Sodium-Induced Colitis Animal Model
4.1. Chemicals
4.2. Animal Care
4.3. Experimental Design
4.4. Evaluation of Disease Activity Index (DAI)
4.5. Histological Evaluation
4.6. Immunohistochemistry
5. Statistical Analysis
6. Results
6.1. GC-MS Analysis of Phytoncide Extract
6.2. Indomethacin-Induced Gastritis Rat Model
6.2.1. Phytoncide Treatment Ameliorates the Severity of Gastric Ulcers in Indomethacin-Induced Gastric Inflammation
6.2.2. Phytoncide Inhibits Histological Changes in Indomethacin-Induced Gastric Inflammation
6.2.3. Phytoncide Suppresses iNOS Expression in Indomethacin-Induced Gastric Damage
6.3. DSS-Induced Colitis Animal Model
6.3.1. Phytoncide Treatment Ameliorates Inflammatory Parameters in DSS-Induced Colitis
6.3.2. Phytoncide Treatment Effects on Histological Changes in DSS-Induced Colitis
6.3.3. Phytoncide Treatment Suppresses iNOS Expression in DSS-Induced Colitis
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Laine, L. Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenerology 2001, 120, 597–606. [Google Scholar] [CrossRef]
- Whitfield-Cargile, C.M.; Cohen, N.D.; Chapkin, R.S.; Weeks, B.R.; Davidson, L.A.; Goldsby, J.S.; Hunt, C.L.; Steinmeyer, S.H.; Menon, R.; Suchodolski, J.S.; et al. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes 2016, 7, 246–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grattan, B.J., Jr.; Bennett, T.; Starks, M.R. Diaphragm disease: NSAID-induced small bowel stricture. Case Rep. Gastroenterol. 2018, 12, 327–330. [Google Scholar] [CrossRef]
- Caron, M.M.J.; Emans, P.J.; Sanen, K.; Surtel, D.A.M.; Cremers, A.; Ophelders, D.; van Rhijn, L.W.; Welting, T.J.M. The role of prostaglandins and COX-enzymes in chondrogenic differentiation of ATDC5 progenitor cells. PLoS ONE 2016, 11, e0153162. [Google Scholar] [CrossRef] [PubMed]
- Rangarajan, S.; Rezonzew, G.; Chumley, P.; Fatima, H.; Golovko, M.Y.; Feng, W.; Hua, P.; Jaimes, E.A. COX-2-derived prostaglandins as mediators of the deleterious effects of nicotine in chronic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 318, F475–F485. [Google Scholar] [CrossRef] [PubMed]
- Shorrock, C.J.; Rees, W.D. Mucosal adaptation to indomethacin induced gastric damage in man-studies on morphology, blood flow, and prostaglandin E2 metabolism. Gut 1992, 33, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Muchekehu, R.W.; Quinton, P.M. A new role for bicarbonate secretion in cervico-uterine mucus release. J. Physiol. 2010, 588, 2329–2342. [Google Scholar] [CrossRef]
- Epa, A.P.; Thatcher, T.H.; Pollock, S.J.; Wahl, L.A.; Lyda, E.; Kottrmann, R.M.; Phipps, R.P.; Sime, P.J. Normal human lung epithelial cells inhibit transforming growth factor-β induced myofibroblast differentiation via prostaglandin E2. PLoS ONE 2015, 10, e135266. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Benard, O.; Syeda, M.M.; Schuster, V.L.; Chi, Y. Inhibition of prostaglandin transporter (PGT) promotes perfusion and vascularization and accelerates wound healing in non-diabetic and diabetic rats. PLoS ONE 2015, 10, e0133615. [Google Scholar] [CrossRef] [PubMed]
- Utzeri, E.; Usai, P. Role of non-steroidal anti-inflammatory drugs on intestinal permeability and nonalcoholic fatty liver disease. World J. Gastroenterol. 2017, 23, 3954–3963. [Google Scholar] [CrossRef]
- Bindu, S.; Mazumder, S.; Bandyopadhyay, U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem. Pharmacol. 2020, 180, 114147. [Google Scholar] [CrossRef]
- Li, Y.; Li, Q.-Y.; So, S.-P.; Ruan, K.-H. A novel single-chain enzyme complex with chain reaction properties rapidly production thromboxane A2 and exhibiting powerful anti-bleeding functions. J. Cell. Mol. Med. 2019, 23, 8343–8354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, A.N.; Daniel, E.F. Nonsteroidal anti-inflammatory drugs, disease-modifying antirheumatic drugs, nonopioid analgesics, & drugs used in gout. In Basic & Clinical Pharmacology, 14th ed.; Bertram, G.K., Ed.; McGraw-Hill Education: New York, NY, USA, 2018; pp. 621–642. [Google Scholar]
- Sartor, R.B. Microbial influences in inflammatory bowel diseases. Gastroenterology 2008, 134, 577–594. [Google Scholar] [CrossRef] [PubMed]
- Ogino, H.; Nakamura, K.; Iwasa, T.; Ihara, E.; Akiho, H.; Motomura, Y.; Akahoshi, H.; Igarashi, H.; Kato, M.; Kotoh, K.; et al. Regulatory T cells expanded by rapamycin in vitro suppress colitis in an experimental mouse model. J. Gastroenterol. 2012, 47, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Aldini, R.; Budriesi, R.; Roda, G.; Micucci, M.; Ioan, P.; D’Errico-Grigioni, A.; Sartini, A.; Guidetti, E.; Marocchi, M.; Cevenini, M.; et al. Curcuma longa extract exerts a myorelaxant effect on the ileum and colon in a mouse experimental colitis model, independent of the anti-inflammatory effect. PLoS ONE 2012, 7, e44650. [Google Scholar] [CrossRef]
- Xavier, R.; Podolsky, D. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007, 448, 427–434. [Google Scholar] [CrossRef]
- Powrie, F. T cells in inflammatory bowel disease: Protective and pathogenic roles. Immunity 1995, 3, 171–174. [Google Scholar] [CrossRef] [Green Version]
- Eichele, D.D.; Kharbanda, K.K. Dextran sodium sulfate colitis murine model: An indispensable tool for advancing our understanding of inflammatory bowel diseases pathogenesis. World J. Gastroenterol. 2017, 23, 6016–6029. [Google Scholar] [CrossRef]
- Blumberg, R.S.; Saubermann, L.J.; Strober, W. Animal models of mucosal inflammation and their relation to human inflammatory bowel disease. Curr. Opin. Immunol. 1999, 11, 648–656. [Google Scholar] [CrossRef]
- Yan, Y.; Kolachala, V.; Dalmasso, G.; Nguyen, H.; Laroui, H.; Sitaraman, S.V.; Merlin, D. Temporal and spatial analysis of clinical and molecular parameters in dextran sodium sulfate induced colitis. PLoS ONE 2009, 4, e6073. [Google Scholar] [CrossRef] [PubMed]
- Rogler, G.; Andus, T. Cytokines in inflammatory bowel disease. World J. Surg. 1998, 22, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Hamamoto, N.; Maemura, K.; Hirata, I.; Murano, M.; Sasaki, S.; Katsu, K. Inhibition of dextran sulphate sodium (DSS)-induced colitis in mice by intracolonically administered antibodies against adhesion molecules (endothelial leucocyte adhesion molecule-1 (ELAM-1) or intercellular adhesion molecule-1 (ICAM-1)). Clin. Exp. Immunol. 1999, 117, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Pinho, R.A.; Silveira, P.C.; Silva, L.A.; Streck, E.L.; Dal-Pizzol, F.; Moreira, J.C. N-acetylcysteine and deferoxamine reduce pulmonary oxidative stress and inflammation in rats after coal dust exposure. Environ. Res. 2005, 99, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Toth, G.F.; Murphy, R.; Lovas, S. Stabilization of local structures by pi-CH and aromatic-backbone amide interactions involving prolyl and aromatic residues. Protein Eng. Des. Sel. 2001, 14, 543–547. [Google Scholar] [CrossRef] [Green Version]
- Wirtz, S.; Neurath, M.F. Mouse models of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2007, 59, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Lee, J.S.; Lee, H.C.; Petriello, M.C.; Kim, B.Y.; Do, J.T.; Lim, D.S.; Lee, H.G.; Han, S.G. Phytoncide extracted from pinecone decreases LPS-induced inflammatory responses in bovine mammary epithelial cells. J. Microbiol. Biotechnol. 2016, 26, 579–587. [Google Scholar] [CrossRef]
- Abe, T.; Hisama, M.; Tanimoto, S.; Shibayama, H.; Mihara, Y.; Nomura, M. Antioxidant effects and antimicrobial activites of phytoncide. Biocontrol. Sci. 2008, 13, 23–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, H.; Cha, B.; Kim, H.; Brito, S.; Kwak, B.M.; Kim, S.T.; Bin, B.-H.; Lee, M.-G. α-pinene enhances the anticancer activity of natural killer cells vis ERK/AKT pathway. Int. J. Mol. Sci. 2021, 22, 656. [Google Scholar] [CrossRef]
- Kim, B.Y.; Lee, C.T. Production of Phytoncide from Korean pine cone waste by steam distillation. J. Ind. Eng. Chem. 2015, 26, 648–658. [Google Scholar] [CrossRef] [Green Version]
- Sreedhar, R.; Arumugam, S.; Thandavarayan, R.A.; Giridharan, V.V.; Karuppagounder, V.; Pitchaimani, V.; Afrin, R.; Harima, M.; Nakamura, T.; Ueno, K.; et al. Toki-shakuyaku-san, a Japanese Kampo medicine, reduces colon inflammation in a mouse model of acute colitis. Int. Immunopharmacol. 2015, 29, 869–875. [Google Scholar] [CrossRef]
- Arumugam, S.; Thandavarayana, R.A.; Pitchaimani, V.; Karuppagounder, V.; Harima, M.; Nishizawa, Y.; Sasaki, K.; Suzuki, K.; Konishi, T.; Watanabe, K. Prevention of DSS induced acute colitis by Petit Vert, a newly developed function improved vegetable, in mice. PharmaNutrition 2014, 2, 129–134. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.W.; Kwon, H.J.; Nam, S.W. Curative effect of selenium against indomethacin-induced gastric ulcers in rats. J. Microbiol. Biotechnol. 2011, 21, 400–404. [Google Scholar] [CrossRef]
- Kim, T.H.; Jeon, E.J.; Cheung, D.Y.; Kim, C.W.; Kim, S.S.; Park, S.H.; Han, S.W.; Kim, M.J.; Lee, Y.S.; Cho, M.L.; et al. Gastroprotective effects of grape seed proanthocyanidin extracts against nonsteroid anti-inflammatory drug-induced gastric injury in rats. Gut Liver 2013, 7, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Murphy, M.P.; Smith, R.A. Drug delivery to mitochondria: The key to mitochondrial medicine. Adv. Drug Deliv. Rev. 2000, 41, 235–250. [Google Scholar] [CrossRef]
- Youn, J.; Lee, J.; Na, H.; Kundu, J.K.; Surh, Y. Resveratrol and piceatannol inhibit iNOS expression and NF-κ B activation in dextran sulfate sodium-induced mouse colitis. Nutr. Cancer 2009, 61, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Hokari, R.; Kato, S.; Matsuzaki, K.; Kuroki, M.; Iwai, A.; Kawaguchi, A.; Nagao, S.; Miyahara, T.; Itoh, K.; Sekizuka, E.; et al. Reduced sensitivity of inducible nitric oxide synthase-deficient mice to chronic colitis. Free Radic. Biol. Med. 2001, 31, 153–163. [Google Scholar] [CrossRef]
- Aoi, Y.; Terashima, S.; Ogura, M.; Nishio, H.; Kato, S.; Takeuchi, K. Roles of nitric oxide (NO) and NO synthases in healing of dextran sulfate sodium-induced rat colitis. J. Physiol. Pharmacol. 2008, 59, 315–336. [Google Scholar] [PubMed]
- Souza, M.H.L.P.; Mota, J.M.S.C.; Oliveira, R.B.; Cunha, F.Q. Gastric damage induced by different doses of indomethacin in rats is variably affected by inhibiting iNOS or leukocyte infiltration. Inflamm. Res. 2008, 57, 28–33. [Google Scholar] [CrossRef]
- Takeuchi, K. Pathogenesis of NSAID-induced gastric damage: Importance of cyclooxygenase inhibition and gastric hypermotility. World J. Gastroenterol. 2012, 18, 2147–2160. [Google Scholar] [CrossRef]
- Camuesco, D.; Comalada, M.; Rodríguez-Cabezas, M.E.; Nieto, A.; Lorente, M.D.; Concha, A.; Zarzuelo, A.; Gálvez, J. The intestinal anti-inflammatory effect of quecitrin is associated with an inhibition in iNOS expression. Br. J. Pharmacol. 2009, 143, 908–918. [Google Scholar] [CrossRef] [Green Version]







| Instrument | Agilent Technologies 7890A, 5975C |
|---|---|
| Column | HP-5MS Column (30 m × 0.25 mm, 0.25 μm) |
| MS ion sourcetemperature | 320 °C |
| Injection porttemperature | 280 °C |
| Carrier gas | He |
| Column flow | 1.5 mL/min |
| Split ratio | Split mode 10:1 |
| Injection volume | 1 μL |
| Oven temp. | 50 °C for 3 min→raised to 150 °C at 2 °C/min→raised to 300 °C at 20 °C/min, held for 10 min |
| Ionization energy | 70 eV |
| Selected ion | α-Pinene: SIM mode m/z 77. 91. 93. 121 Scan mode 40–600 amu |
| A | No. | Retention Time (min) | Name | Area (%) |
| 1 | 8.392 | Alpha-Pinene | 49.431 | |
| 2 | 8.942 | Camphene | 1.442 | |
| 3 | 10.007 | Beta-Pinene | 13.247 | |
| 4 | 10.697 | Beta-Myrcene | 3.689 | |
| 5 | 11.189 | Bicyclo(4.1.0) hept-3-ene | 3.067 | |
| 6 | 11.997 | Limonene | 27.118 | |
| 7 | 23.280 | 1,4-Methanoazulene | 1.217 | |
| 8 | 23.572 | Caryophyllene | 0.789 | |
| B | No. | Retention Time (min) | Name | Area (%) |
| 1 | 18.96 | Verbenone | 9.230 | |
| 2 | 18.66 | Alpha-terpinieol | 8.110 | |
| 3 | 16 | Fenchol | 6.830 | |
| 4 | 14.36 | Camphor | 4.750 | |
| 5 | 18.74 | Borneol | 4.660 | |
| 6 | 12.35 | p-Alpha-dimethylstyrene | 4.430 | |
| 7 | 20.62 | Myrtenol | 3.970 | |
| 8 | 21.46 | Carveol | 3.860 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memon, A.; Kim, B.Y.; Kim, S.-e.; Pyao, Y.; Lee, Y.-G.; Kang, S.C.; Lee, W.K. Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation. Molecules 2021, 26, 1895. https://doi.org/10.3390/molecules26071895
Memon A, Kim BY, Kim S-e, Pyao Y, Lee Y-G, Kang SC, Lee WK. Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation. Molecules. 2021; 26(7):1895. https://doi.org/10.3390/molecules26071895
Chicago/Turabian StyleMemon, Azra, Bae Yong Kim, Se-eun Kim, Yuliya Pyao, Yeong-Geun Lee, Se Chan Kang, and Woon Kyu Lee. 2021. "Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation" Molecules 26, no. 7: 1895. https://doi.org/10.3390/molecules26071895
APA StyleMemon, A., Kim, B. Y., Kim, S. -e., Pyao, Y., Lee, Y. -G., Kang, S. C., & Lee, W. K. (2021). Anti-Inflammatory Effect of Phytoncide in an Animal Model of Gastrointestinal Inflammation. Molecules, 26(7), 1895. https://doi.org/10.3390/molecules26071895