Smart, Naturally-Derived Macromolecules for Controlled Drug Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of pH-Responsive Troxerutin-Based Star-Shaped Polymers with Different Arm Lengths
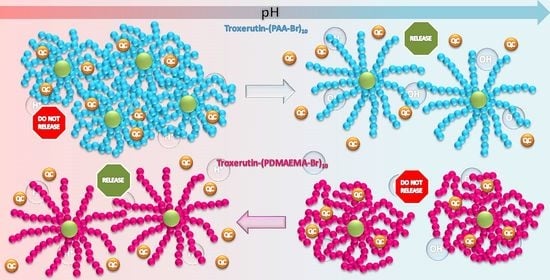
2.2. Stimuli-Responsive Behavior of Troxerutin-Based Polymers
2.3. In Vitro Drug Release
3. Materials and Methods
3.1. Chemicals
3.2. Analysis
3.3. Synthesis of Troxerutin-Based Macroinitiator (Trox-Br10)
3.4. General Procedure for SARA ATRP of tBA from Trox-Br10
3.5. Transformation of PtBA to PAA Side Chains
3.6. General Procedure for SARA ATRP of DMAEMA from Trox-Br10
3.7. Determination of pH-sensitivity of PAA- and PDMAEMA-Based Polymers
3.8. Determination of Thermoresponsive Behavior of PDMAEMA-Based Polymers
3.9. Loading of Quercetin into the PAA- and PDMAEMA-Based Polymer and Release Behavior upon pH Changes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hunter, A.C.; Moghimi, S.M. Smart polymers in drug delivery: A biological perspective. Polym. Chem. 2017, 8, 41–51. [Google Scholar] [CrossRef] [Green Version]
- Wells, C.M.; Harris, M.; Choi, L.; Murali, V.P.; Guerra, F.D.; Jennings, J.A. Stimuli-responsive drug release from smart polymers. J. Funct. Biomater. 2019, 10, 34. [Google Scholar] [CrossRef] [Green Version]
- Sponchioni, M.; Capasso Palmiero, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yan, J.; Avellan, A.; Gao, X.; Matyjaszewski, K.; Tilton, R.D.; Lowry, G.V. Temperature- and pH-responsive star polymers as nanocarriers with potential for in vivo agrochemical delivery. ACS Nano 2020, 14, 10954–10965. [Google Scholar] [CrossRef]
- Zaborniak, I.; Macior, A.; Chmielarz, P. Stimuli-responsive rifampicin-based macromolecules. Materials 2020, 13, 3843. [Google Scholar] [CrossRef]
- Pack, D.W.; Hoffman, A.S.; Pun, S.; Stayton, P.S. Design and development of polymers for gene delivery. Nat. Rev. Drug Discov. 2005, 4, 581–593. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.G.; Oo, N.N.L.; Lee, J.P.; Li, Z.B.; Loh, X.J. Recent development of synthetic nonviral systems for sustained gene delivery. Drug Discov. Today 2017, 22, 1318–1335. [Google Scholar] [CrossRef] [PubMed]
- Jabbarzadeh, A.; Atkinson, J.D.; Tanner, R.I. Effect of molecular shape on rheological properties in molecular dynamics simulation of star, H, comb, and linear polymer melts. Macromolecules 2003, 36, 5020–5031. [Google Scholar] [CrossRef]
- Xu, J.; Ge, Z.; Zhu, Z.; Luo, S.; Liu, H.; Liu, S. Synthesis and micellization properties of double hydrophilic A2BA2 and A4BA4 non-linear block copolymers. Macromolecules 2006, 39, 8178–8185. [Google Scholar] [CrossRef]
- Ge, Z.; Cai, Y.; Yin, J.; Zhu, Z.; Rao, J.; Liu, S. Synthesis and ‘schizophrenic’ micellization of double hydrophilic AB4 miktoarm star and ab diblock copolymers: Structure and kinetics of micellization. Langmuir 2007, 23, 1114–1122. [Google Scholar] [CrossRef]
- Jiang, X.; Lu, G.; Feng, C.; Li, Y.; Huang, X. Poly(acrylic acid)-graft-poly(N-vinylcaprolactam): A novel pH and thermo dual-stimuli responsive system. Polym. Chem. 2013, 4, 3876–3884. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, J.; Wang, A. Preparation and characterization of a novel pH-sensitive chitosan-g-poly (acrylic acid)/attapulgite/sodium alginate composite hydrogel bead for controlled release of diclofenac sodium. Carbohydr. Polym. 2009, 78, 731–737. [Google Scholar] [CrossRef]
- Hu, X.; Wei, W.; Qi, X.; Yu, H.; Feng, L.; Li, J.; Wang, S.; Zhang, J.; Dong, W. Preparation and characterization of a novel pH-sensitive Salecan-g-poly(acrylic acid) hydrogel for controlled release of doxorubicin. J. Mater. Chem. B 2015, 3, 2685–2697. [Google Scholar] [CrossRef]
- Xu, M.; Zhu, J.; Wang, F.; Xiong, Y.; Wu, Y.; Wang, Q.; Weng, J.; Zhang, Z.; Chen, W.; Liu, S. Improved in vitro and in vivo biocompatibility of graphene oxide through surface modification: Poly(acrylic acid)-functionalization is superior to PEGylation. ACS Nano 2016, 10, 3267–3281. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-C.; Xu, X.-D.; Chen, C.-S.; Wang, G.-R.; Wang, B.; Zhang, X.-Z.; Zhuo, R.-X. Study on novel hydrogels based on thermosensitive PNIPAAm with pH sensitive PDMAEMA grafts. Colloids Surf. B 2008, 67, 245–252. [Google Scholar] [CrossRef]
- Zhou, Z.; Guo, F.; Wang, N.; Meng, M.; Li, G. Dual pH-sensitive supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2018, 116, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Yuan, W. CO2- and thermo-responsive vesicles: From expansion–contraction transformation to vesicles-micelles transition. Polym. Chem. 2015, 6, 2457–2465. [Google Scholar] [CrossRef]
- Bonkovoski, L.C.; Martins, A.F.; Bellettini, I.C.; Garcia, F.P.; Nakamura, C.V.; Rubira, A.F.; Muniz, E.C. Polyelectrolyte complexes of poly[(2-dimethylamino) ethyl methacrylate]/chondroitin sulfate obtained at different pHs: Preparation, characterization, cytotoxicity and controlled release of chondroitin sulfate. J. Control. Release 2015, 213, e29–e30. [Google Scholar] [CrossRef]
- Ferjaoui, Z.; Jamal Al Dine, E.; Kulmukhamedova, A.; Bezdetnaya, L.; Soon Chang, C.; Schneider, R.; Mutelet, F.; Mertz, D.; Begin-Colin, S.; Quilès, F.; et al. Doxorubicin-loaded thermoresponsive superparamagnetic nanocarriers for controlled drug delivery and magnetic hyperthermia applications. ACS Appl. Mater. Interfaces 2019, 11, 30610–30620. [Google Scholar] [CrossRef]
- Almeida, E.A.M.S.; Bellettini, I.C.; Garcia, F.P.; Farinácio, M.T.; Nakamura, C.V.; Rubira, A.F.; Martins, A.F.; Muniz, E.C. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017, 171, 259–266. [Google Scholar] [CrossRef]
- Huang, B.; Chen, F.; Shen, Y.; Qian, K.; Wang, Y.; Sun, C.; Zhao, X.; Cui, B.; Gao, F.; Zeng, Z.; et al. Advances in targeted pesticides with environmentally responsive controlled release by nanotechnology. Nanomaterials 2018, 8, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mann, M.; Kruger, J.E.; Andari, F.; McErlean, J.; Gascooke, J.R.; Smith, J.A.; Worthington, M.J.H.; McKinley, C.C.C.; Campbell, J.A.; Lewis, D.A.; et al. Sulfur polymer composites as controlled-release fertilisers. Org. Biomol. Chem. 2019, 17, 1929–1936. [Google Scholar] [CrossRef]
- Siegwart, D.J.; Oh, J.K.; Matyjaszewski, K. ATRP in the design of functional materials for biomedical applications. Prog. Polym. Sci. 2012, 37, 18–37. [Google Scholar] [CrossRef] [Green Version]
- Matyjaszewski, K. Advanced materials by atom transfer radical polymerization. Adv. Mater. 2018, 30, 1706441. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Modification of wood-based materials by atom transfer radical polymerization methods. Eur. Polym. J. 2019, 120, 109253. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Matyjaszewski, K. Synthesis of riboflavin-based macromolecules through low ppm ATRP in aqueous media. Macromol. Chem. Phys. 2020, 221, 1900496. [Google Scholar] [CrossRef]
- Corrigan, N.; Jung, K.; Moad, G.; Hawker, C.J.; Matyjaszewski, K.; Boyer, C. Reversible-deactivation radical polymerization (Controlled/living radical polymerization): From discovery to materials design and applications. Prog. Polym. Sci. 2020, 111, 101311. [Google Scholar] [CrossRef]
- Park, S.; Chmielarz, P.; Gennaro, A.; Matyjaszewski, K. Simplified electrochemically mediated atom transfer radical polymerization using a sacrificial anode. Angew. Chem. Int. Ed. 2015, 54, 2388–2392. [Google Scholar] [CrossRef]
- Beers, K.L. The first dive into the mechanism and kinetics of ATRP. Macromolecules 2020, 53, 1115–1118. [Google Scholar] [CrossRef] [Green Version]
- Lorandi, F.; Matyjaszewski, K. Why do we need more active ATRP catalysts? Isr. J. Chem. 2020, 60, 108–123. [Google Scholar] [CrossRef] [Green Version]
- Doerr, A.M.; Burroughs, J.M.; Gitter, S.R.; Yang, X.; Boydston, A.J.; Long, B.K. Advances in polymerizations modulated by external stimuli. ACS Catal. 2020, 10, 14457–14515. [Google Scholar] [CrossRef]
- Chmielarz, P.; Park, S.; Sobkowiak, A.; Matyjaszewski, K. Synthesis of β-cyclodextrin-based star polymers via a simplified electrochemically mediated ATRP. Polymer 2016, 88, 36–42. [Google Scholar] [CrossRef] [Green Version]
- Li, R.; Li, X.; Zhang, Y.; Delawder, A.O.; Colley, N.D.; Whiting, E.A.; Barnes, J.C. Diblock brush-arm star copolymers via a core-first/graft-from approach using γ-cyclodextrin and ROMP: A modular platform for drug delivery. Polym. Chem. 2020, 11, 541–550. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P.; Martinez, M.R.; Wolski, K.; Wang, Z.; Matyjaszewski, K. Synthesis of high molecular weight poly(n-butyl acrylate) macromolecules via seATRP: From polymer stars to molecular bottlebrushes. Eur. Polym. J. 2020, 126, 109566. [Google Scholar] [CrossRef]
- Zaborniak, I.; Chmielarz, P. Miniemulsion switchable electrolysis under constant current conditions. Polym. Adv. Technol. 2020, 31, 2806–2815. [Google Scholar] [CrossRef]
- Vinothkumar, R.; Kumar, R.V.; Sudha, M.; Viswanathan, P.; Balasubramanian, T.; Nalini, N. Modulatory effect of troxerutin on biotransforming enzymes and preneoplasic lesions induced by 1,2-dimethylhydrazine in rat colon carcinogenesis. Exp. Mol. Pathol. 2014, 96, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Panat, N.A.; Singh, B.G.; Maurya, D.K.; Sandur, S.K.; Ghaskadbi, S.S. Troxerutin, a natural flavonoid binds to DNA minor groove and enhances cancer cell killing in response to radiation. Chem. Biol. Interact. 2016, 251, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Chmielarz, P. Synthesis of α-D-glucose-based star polymers through simplified electrochemically mediated ATRP. Polymer 2016, 102, 192–198. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Park, S.; Matyjaszewski, K. PEO-b-PNIPAM copolymers via SARA ATRP and eATRP in aqueous media. Polymer 2015, 71, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Chmielarz, P.; Sobkowiak, A.; Matyjaszewski, K. A simplified electrochemically mediated ATRP synthesis of PEO-b-PMMA copolymers. Polymer 2015, 77, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Laguecir, A.; Ulrich, S.; Labille, J.; Fatin-Rouge, N.; Stoll, S.; Buffle, J. Size and pH effect on electrical and conformational behavior of poly(acrylic acid): Simulation and experiment. Eur. Polym. J. 2006, 42, 1135–1144. [Google Scholar] [CrossRef]
- Zhang, L.; Eisenberg, A. Multiple morphologies and characteristics of “crew-cut” micelle-like aggregates of polystyrene-b-poly(acrylic acid) diblock copolymers in aqueous solutions. J. Am. Chem. Soc. 1996, 118, 3168–3181. [Google Scholar] [CrossRef]
- Strandman, S.; Hietala, S.; Aseyev, V.; Koli, B.; Butcher, S.J.; Tenhu, H. Supramolecular assemblies of amphiphilic PMMA-block-PAA stars in aqueous solutions. Polymer 2006, 47, 6524–6535. [Google Scholar] [CrossRef]
- Fresnais, J.; Yan, M.; Courtois, J.; Bostelmann, T.; Bée, A.; Berret, J.F. Poly(acrylic acid)-coated iron oxide nanoparticles: Quantitative evaluation of the coating properties and applications for the removal of a pollutant dye. J. Colloid Interface Sci. 2013, 395, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Wang, J.; Zhang, H.; Jiang, G.; Kan, C. Synthesis and characterization of monodispersed P(St-co-DMAEMA) nanoparticles as pH-sensitive drug delivery system. Mater. Sci. Eng. C 2014, 45, 1–7. [Google Scholar] [CrossRef]
- Bao, H.; Li, L.; Gan, L.H.; Ping, Y.; Li, J.; Ravi, P. Thermo- and pH-responsive association behavior of dual hydrophilic graft chitosan terpolymer synthesized via ATRP and click chemistry. Macromolecules 2010, 43, 5679–5687. [Google Scholar] [CrossRef]
- Xiao, G.; Hu, Z.; Zeng, G.; Wang, Y.; Huang, Y.; Hong, X.; Xia, B.; Zhang, G. Effect of hydrophilic chain length on the aqueous solution behavior of block amphiphilic copolymers PMMA-b-PDMAEMA. J. Appl. Polym. Sci. 2012, 124, 202–208. [Google Scholar] [CrossRef]
- Wright, D.B.; Patterson, J.P.; Pitto-Barry, A.; Cotanda, P.; Chassenieux, C.; Colombani, O.; O’Reilly, R.K. Tuning the aggregation behavior of pH-responsive micelles by copolymerization. Polym. Chem. 2015, 6, 2761–2768. [Google Scholar] [CrossRef] [Green Version]
- Fournier, D.; Hoogenboom, R.; Thijs, H.M.L.; Paulus, R.M.; Schubert, U.S. Tunable pH- and temperature-sensitive copolymer libraries by reversible addition−fragmentation chain transfer copolymerizations of methacrylates. Macromolecules 2007, 40, 915–920. [Google Scholar] [CrossRef]
- Ahmad, Z.; Shah, A.; Siddiq, M.; Kraatz, H.-B. Polymeric micelles as drug delivery vehicles. RSC Adv. 2014, 4, 17028–17038. [Google Scholar] [CrossRef]
- Dian, L.; Yu, E.; Chen, X.; Wen, X.; Zhang, Z.; Qin, L.; Wang, Q.; Li, G.; Wu, C. Enhancing oral bioavailability of quercetin using novel soluplus polymeric micelles. Nanoscale Res. Lett. 2014, 9, 684. [Google Scholar] [CrossRef] [Green Version]
- Kaur, A.; Ribelli, T.G.; Schröder, K.; Matyjaszewski, K.; Pintauer, T. Properties and ATRP activity of copper complexes with substituted tris(2-pyridylmethyl)amine-based ligands. Inorg. Chem. 2015, 54, 1474–1486. [Google Scholar] [CrossRef]
- Chmielarz, P.; Krys, P.; Wang, Z.; Wang, Y.; Matyjaszewski, K. Synthesis of well-defined polymer brushes from silicon wafers via surface-initiated seATRP. Macromol. Chem. Phys. 2017, 218, 1700106. [Google Scholar] [CrossRef]







| Entry | Monomer | DPtarget | Conv 1 (%) | kpapp 2 (h−1) | DPn,theo 1 (per chain) | Mn,theo3 (×10−3) | Mn,app4 (×10−3) | Mw/Mn4 | dnumber5 (nm) | dnumber6 (nm) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | tBA | 50 | 79 | 0.594 | 40 | 53.0 | 39.5 | 1.05 | 7.7 ± 0.7 | 3.4 ± 0.3 |
| 2 | tBA | 100 | 67 | 0.609 | 67 | 88.6 | 66.7 | 1.05 | 10.6 ± 0.7 | 4.3 ± 0.2 |
| 3 | tBA | 200 | 78 | 0.734 | 157 | 203.3 | 131.4 | 1.09 | 17.1 ± 1.8 | 6.6 ± 0.7 |
| 4 | DMAEMA | 800 | 12 | 1.723 | 98 | 156.7 | 201.9 | 1.71 | - | 7.2 ± 0.9 7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborniak, I.; Macior, A.; Chmielarz, P. Smart, Naturally-Derived Macromolecules for Controlled Drug Release. Molecules 2021, 26, 1918. https://doi.org/10.3390/molecules26071918
Zaborniak I, Macior A, Chmielarz P. Smart, Naturally-Derived Macromolecules for Controlled Drug Release. Molecules. 2021; 26(7):1918. https://doi.org/10.3390/molecules26071918
Chicago/Turabian StyleZaborniak, Izabela, Angelika Macior, and Paweł Chmielarz. 2021. "Smart, Naturally-Derived Macromolecules for Controlled Drug Release" Molecules 26, no. 7: 1918. https://doi.org/10.3390/molecules26071918
APA StyleZaborniak, I., Macior, A., & Chmielarz, P. (2021). Smart, Naturally-Derived Macromolecules for Controlled Drug Release. Molecules, 26(7), 1918. https://doi.org/10.3390/molecules26071918