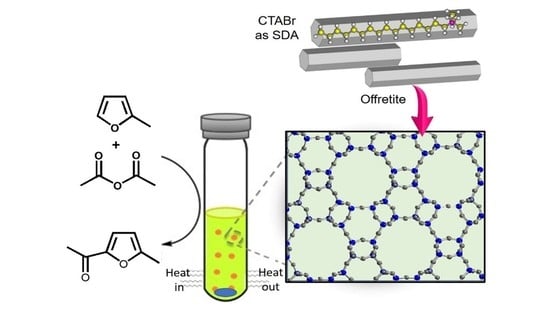
Offretite Zeolite Single Crystals Synthesized by Amphiphile-Templating Approach
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Materials
3.2. Synthesis of CTA+-Offretite Zeolite
3.3. Characterization
3.4. Catalytic Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dusselier, M.; Davis, M.E. Small-Pore Zeolites: Synthesis and Catalysis. Chem. Rev. 2018, 118, 5265–5329. [Google Scholar] [CrossRef]
- Wong, S.-F.; Deekomwong, K.; Wittayakun, J.; Ling, T.C.; Muraza, O.; Adam, F.; Ng, E.-P. Crystal growth study of KF nanozeolite and its catalytic behavior in Aldol condensation of benzaldehyde and heptanal enhanced by microwave heating. Mater. Chem. Phys. 2017, 196, 295–301. [Google Scholar] [CrossRef]
- Liu, J.; Luo, W.; Cao, H.; Weng, L.; Feng, G.; Fu, X.-Z.; Luo, J.-L. Understanding the immobilization mechanisms of hazardous heavy metal ions in the cage of solidate at molecular level: A DTF study. Microporous Mesoporous Mater. 2020, 306, 110409. [Google Scholar] [CrossRef]
- Tan, K.-H.; Awala, H.; Mukti, R.R.; Wong, K.-L.; Ling, T.C.; Mintova, S.; Ng, E.-P.; Taiwan, J. Zeolite nanoparticles as effective antioxidant additive for the preservation of palm oil-based lubricant. Inst. Chem. Eng. 2016, 58, 565–571. [Google Scholar] [CrossRef]
- International Zeolite Association. Available online: http://www.iza-online.org/ (accessed on 21 March 2021).
- Catizzone, E.; Migliori, M.; Mineva, T.; van Daele, S.; Valtchev, V.; Girodano, G. New synthesis routes and catalytic applications of ferrierite crystals. Part 2:1,8-Diaminooctane as a new OSDA. Microporous Mesoporous Mater. 2020, 296, 109987. [Google Scholar] [CrossRef]
- Tosheva, L.; Ng, E.-P.; Mintova, S.; Hölzl, M.; Metzger, T.H.; Doyle, A.M. AlPO4-18 Seed Layers and Films by Secondary Growth. Chem. Mater. 2008, 20, 5721–5726. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Q.; Meng, X.; Xiao, F.-S. Insights into the Organotemplate-Free Synthesis of Zeolite Catalysts. Engineering 2017, 3, 567–574. [Google Scholar] [CrossRef]
- Ng, E.-P.; Awala, H.; Ghoy, J.-P.; Vicente, A.; Ling, T.C.; Ng, Y.H.; Mintova, S.; Adam, F. Effects if ultrasonic irradiation on crystallization and structural properties of EMT-tyoe zeolite nanocrystals. Mater. Chem. Phys. 2015, 159, 38–45. [Google Scholar] [CrossRef]
- Khoo, D.Y.; Kok, W.-M.; Mukti, R.R.; Mintova, S.; Ng, E.-P. Ionothermal approach for synthesizing AlPO-5 with hexagonal thin-plate morphology influenced by various parameters at ambient pressure. Solid State Sci. 2013, 25, 63–69. [Google Scholar] [CrossRef]
- Ng, E.-P.; Itani, L.; Sekhon, S.S.; Mintova, S. Micro- to Macroscopic Observations of MnAlPO-5 Nanocrystal Growth in Ionic-Liquid Media. Chem. Eur. J. 2010, 16, 12890–12897. [Google Scholar] [CrossRef] [PubMed]
- Itakuram, M.; Ota, K.; Shibata, S.; Inoue, T.; Ide, Y.; Sadakane, M.; Sano, T. Influence of starting zeolite on synthesis of RUT type zeolite by interzeolite conversion method. J. Cryst. Growth 2011, 314, 274–278. [Google Scholar] [CrossRef]
- Boruntea, C.-R.; Lundegaard, L.F.; Corma, A.; Vennestrom, P.N.R. Crystallization of AEI and AFX zeolites through zeolite-to-zeolite transformations. Microporous Mesoporous Mater. 2019, 278, 105–114. [Google Scholar] [CrossRef]
- Trachta, M.; Nachtigall, P.; Bludsky, O. The ADOR synthesis of new zeolites: In silico investigation. Catal. Today 2015, 243, 32–38. [Google Scholar] [CrossRef]
- Prech, J.; Cejka, J. UTL titanosilicate: An extra-large pore epoxidation catalyst with tunable textural properties. Catal. Today 2016, 277, 2–8. [Google Scholar] [CrossRef]
- Caullet, P.; Paillaud, J.-L.; Masseron, A.S.; Soulard, M.; Patarin, J. The fluoride route: A strategy to crystalline porous materials. CR Chim. 2005, 8, 245–266. [Google Scholar] [CrossRef]
- Matijasic, A.; Gramlich, V.; Patarin, J. Mu-17: A new member of the gallophosphate family prepared by the fluoride route. Solid State Sci. 2001, 3, 155–167. [Google Scholar] [CrossRef]
- Gard, J.A.; Tait, J.M. The crystal structure of the zeolite offretite, K1.1Ca1.1Mg0.7[Si12.8Al5.2O36].15.2H2O. Acta Cryst. 1972, B28, 825–834. [Google Scholar] [CrossRef]
- Lukaszuk, K.A.; Gama, D.R.; Odegaard, S.O.; Lazzarini, A.; Berlier, G.; Bordiga, S.; Lillerud, K.P.; Olsbye, U.; Beato, P.; Lundegaard, L.F.; et al. Zeolite morphology and catalyst performance: Conversion of methanol to hydrocarbons over offretite. Catal. Sci. Technol. 2017, 7, 5435–5447. [Google Scholar] [CrossRef]
- Sanyuan, Y.; Evmiridis, N.P. Synthesis and characterization of an offretite/erionite type zeolite. Micropor. Mater. 1996, 6, 19–26. [Google Scholar]
- Gao, F.; Li, X.; Zhu, G.; Qui, S.; Wei, B.; Shao, C.; Terasaki, O. The synthesis of offretite single crystals in the system containing pyrocatechol or F−. Mater. Lett. 2001, 48, 1–7. [Google Scholar] [CrossRef]
- Matijasic, A.; Patarin, J. Synthesis of OFF-type zeolite in a quasi non aqueous medium: Structure directing role of p-dioxane and alkaline cations. Microporous Mesoporous Mater. 1999, 29, 405–412. [Google Scholar] [CrossRef]
- Itakura, M.; Oumi, Y.; Sadakane, M.; Sano, T. Synthesis of high-silica offretite by the interzeolite conversion method. Mater. Res. Bull. 2020, 45, 646–650. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Adam, F.; Appaturi, J.N.; Ng, E.-P. Halide aided synergistic ring opening mechanism of epoxides and their cycloaddition to CO2 using MSM-41-imidazolium bromide catalyst. J. Mol. Catal. A Chem. 2014, 386, 42–48. [Google Scholar] [CrossRef]
- Chen, H.; Fu, S.; Fu, L.; Yang, H.; Chen, D. Simple Synthesis and Characterization of Hexagonal and Ordered Al-MCM-41 from Natural Perlite. Minerals 2019, 9, 264. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Karakilic, P.; Liu, X.; Shan, M.; Nijmeijer, A.; Winnubst, L.; Gascon, J.; Kapteign, F. One-Pot Synthesis of High-Flux b-Oriented MFI Zeolite Membranes for Xe Recovery. ACS Appl. Mater. Interfaces 2018, 10, 33574–33580. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.-F.; Awala, H.; Vincente, A.; Retoux, R.; Ling, T.C.; Mintova, S.; Mukti, R.R.; Ng, E.-P. K-F zeolite nanocrystals synthesized from organic-template-free precursor mixture. Microporous Mesoporous Mater. 2017, 249, 105–110. [Google Scholar] [CrossRef]
- Ng, E.-P.; Delmotte, L.; Mintova, S. Environmentally benign synthesis of nanosized aluminophosphate enhanced by microwave heating. Green Chem. 2008, 10, 1043–1048. [Google Scholar] [CrossRef]
- The Molecular Sizes of the Compounds Were Estimated Using HyperChem™-Release 8.0 for Windows Molecular Modeling System; Hypercube, Inc.: Gainesville, FL, USA, 2011.
- Ali, D.; Zeiger, C.R.; Azim, M.M.; Lein, H.L.; Mathisen, K. Evaluation of surfactant templates for one-pot hydrothermal synthesis of hierarchical SAPO-5. Microporous Mesoporous Mater. 2020, 306, 110364. [Google Scholar] [CrossRef]
- Přech, J.; Bozhilov, K.N.; Fallah, J.E.; Barrier, N.; Valtchev, V. Fluoride etching opens the structure and strengthens the active sites of the layered ZSM-5 zeolite. Microporous Mesoporous Mater. 2019, 280, 297–305. [Google Scholar] [CrossRef]
- Ghrear, T.M.A.; Rigolet, S.; Daou, T.J.; Mintova, S.; Ling, T.C.; Tan, S.H.; Ng, E.P. Synthesis of Cs-ABW nanozeolite in organotemplate-free system. Microporous Mesoporous Mater. 2019, 227, 78–83. [Google Scholar] [CrossRef]
- Serrano, D.P.; Escola, J.M.; Pizarro, P. Synthesis strategies in the search for hierarchical zeolites. Chem. Soc. Rev. 2013, 42, 4004–4035. [Google Scholar] [CrossRef] [PubMed]
- Mintova, S. Verified Syntheses of Zeolitic Materials, 3rd ed.; Elsevier Science: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Ma, Y.-K.; Rigolet, S.; Michelin, L.; Paillaud, J.L.; Mintova, S.; Khoerunnisa, F.; Daou, T.J.; Ng, E.-P. Facile and fast determination of Si/Al ratio of zeolites using FTIR spectroscopy technique. Microporous Mesoporous Mater. 2021, 311, 110683. [Google Scholar] [CrossRef]





| Samples | Si/Al | SBET (m2/g) | SMic (m2/g) | SExt (m2/g) | VMeso (cm3/g) | VTot (cm3/g) | NH3-TPD Acidity (mmol/g) | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Tdes, 150 °C | Tdes, 200 °C | Tdes, 350 °C | Total | |||||||
| CTA+-offretite | 4.1 | 523 | 503 | 20 | 0.05 | 0.26 | 0.18 | 0.24 | 0.32 | 0.74 |
| TMA+-offretite | 3.5 | 504 | 491 | 13 | 0 | 0.19 | 0.17 | 0.26 | 0.25 | 0.68 |
| Entry | Catalysts | Conversion (%) | ||
|---|---|---|---|---|
| 10 min | 20 min | 40 min | ||
| 1 | No catalyst | 3.2 | 6.4 | 12.0 |
| 2 | CTA+-offretite | 52.6 | 72.3 | 83.5 |
| 3 | CTA+-offretite a | 50.2 | 68.0 | 79.1 |
| 4 | TMA+-offretite | 44.7 | 61.7 | 78.3 |
| 5 | HCl | 35.7 | 55.3 | 72.3 |
| 6 | HNO3 | 51.1 | 71.9 | 83.6 |
| 7 | CH3COOH | 16.0 | 31.9 | 47.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, E.-P.; Ahmad, N.H.; Khoerunnisa, F.; Mintova, S.; Ling, T.C.; Daou, T.J. Offretite Zeolite Single Crystals Synthesized by Amphiphile-Templating Approach. Molecules 2021, 26, 2238. https://doi.org/10.3390/molecules26082238
Ng E-P, Ahmad NH, Khoerunnisa F, Mintova S, Ling TC, Daou TJ. Offretite Zeolite Single Crystals Synthesized by Amphiphile-Templating Approach. Molecules. 2021; 26(8):2238. https://doi.org/10.3390/molecules26082238
Chicago/Turabian StyleNg, Eng-Poh, Nur Hidayahni Ahmad, Fitri Khoerunnisa, Svetlana Mintova, Tau Chuan Ling, and T. Jean Daou. 2021. "Offretite Zeolite Single Crystals Synthesized by Amphiphile-Templating Approach" Molecules 26, no. 8: 2238. https://doi.org/10.3390/molecules26082238
APA StyleNg, E. -P., Ahmad, N. H., Khoerunnisa, F., Mintova, S., Ling, T. C., & Daou, T. J. (2021). Offretite Zeolite Single Crystals Synthesized by Amphiphile-Templating Approach. Molecules, 26(8), 2238. https://doi.org/10.3390/molecules26082238