Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants
Abstract
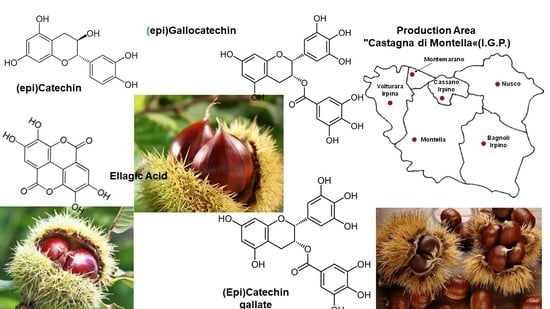
:1. Introduction
2. Results
2.1. Polyphenol Extraction
2.2. MALDI-TOF-MS Profiling of Polyphenols from Chestnut Pericarp and Integument Tissues
2.3. ESI-qTOF-MS Analysis of Polyphenols from Chestnut Pericarp and Integument Tissues
2.4. Time-Course Analysis of Total Phenols Released in Wastewater from Different Chestnut Water Curing Treatments
2.5. Mass Spectrometric Analysis of Polyphenols Released in Wastewater from Different Chestnut Water Curing Treatments
3. Materials and Methods
3.1. Chestnut Sampling and Treatments
3.2. Quantitative Evaluation of Total Phenolic Compounds in Wastewaters
3.3. Extraction of Polyphenols from Chestnut Integument, Pericarp and Curing Wastewaters
3.4. MALDI-TOF-MS Analysis
3.5. ESI-qTOF-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Barreira, J.C.M.; Ferreira, I.C.F.R.; Oliveira, M.B.P.P.; Pereira, J.A. Antioxidant Activities of the Extracts from Chestnut Flower, Leaf, Skins and Fruit. Food Chem. 2008, 107, 1106–1113. [Google Scholar] [CrossRef]
- Gonçalves, B.; Borges, O.; Costa, H.S.; Bennett, R.; Santos, M. Silva Metabolite Composition of Chestnut (Castanea Sativa Mill.) Upon Cooking: Proximate Analysis, Fibre, Organic Acids and Phenolics. Food Chem. 2010, 122, 154–160. [Google Scholar] [CrossRef]
- Hu, M.; Yang, X.; Chang, X. Bioactive phenolic components and potential health effects of chestnut shell: A review. J. Food Biochem. 2021, e13696. [Google Scholar] [CrossRef]
- Sorice, A.; Siano, F.; Capone, F.; Guerriero, E.; Picariello, G.; Budillon, A.; Ciliberto, G.; Paolucci, M.; Costantini, S.; Volpe, M.G. Potential Anticancer Effects of Polyphenols from Chestnut Shell Extracts: Modulation of Cell Growth, and Cytokinomic and Metabolomic Profiles. Molecules 2016, 21, 1411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coccia, E.; Siano, F.; Volpe, M.G.; Varricchio, E.; Eroldogan, O.T.; Paolucci, M. Chestnut Shell Extract Modulates Immune Parameters in the Rainbow Trout Oncorhynchus Mykiss. Fishes 2019, 4, 18. [Google Scholar] [CrossRef] [Green Version]
- Vamanu, E.; Gatea, F.; Pelinescu, D.R. Bioavailability and Bioactivities of Polyphenols Eco Extracts from Coffee Grounds after In Vitro Digestion. Foods 2020, 9, 1281. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and Health: Moving Beyond Antioxidants. J. Berry Res. 2011, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.; Zhao, S.; Chen, S.; Liu, J.; Shi, L.; Wang, X.; Liu, Y.; Ma, C. Hypoglycemic and Hypolipidemic Effects of Polyphenols from Burs of Castanea Mollissima Blume. Molecules 2011, 16, 9764–9774. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jovanovic, J.A.; Mihailovic, M.; Uskokovic, A.S.; Grdovic, N.; Dinic, S.; Poznanovic, G.; Mujic, I.; Vidakovic, M. Evaluation of the Antioxidant and Antiglycation Effects of Lactarius Deterrimus and Castanea Sativa Extracts on Hepatorenal Injury in Streptozotocin-Induced Diabetic Rats. Front. Pharm. 2017, 8, 793. [Google Scholar] [CrossRef] [Green Version]
- Serafini, M.; Peluso, I. Functional Foods for Health: The Interrelated Antioxidant and Anti-Inflammatory Role of Fruits, Vegetables, Herbs, Spices and Cocoa in Humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [Green Version]
- Vazquez, G.; Pizzi, A.; Freire, M.; Santos, J.; Antorrena, G.; González-Álvarez, J. Maldi-Tof, Hplc-Esi-Tof and 13c-Nmr Characterization of Chestnut (Castanea Sativa) Shell Tannins for Wood Adhesives. Wood Sci. Technol. 2013, 47, 523–535. [Google Scholar] [CrossRef]
- Vasconcelos, M.C.B.M.; Richard, N.B.; Quideau, S.; Jacquet, R.; Rosa, E.A.S.; Ferreira-Cardoso, J.V. Evaluating the Potential of Chestnut (Castanea Sativa Mill.) Fruit Pericarp and Integument as a Source of Tocopherols, Pigments and Polyphenols. Ind. Crops Prod. 2010, 31, 301–311. [Google Scholar] [CrossRef]
- Santos, J.; Antorrena, G.; Freire, M.S.; Pizzi, A.; González-Álvarez, J. Environmentally Friendly Wood Adhesives Based on Chestnut (Castanea Sativa) Shell Tannins. Eur. J. Wood Wood Prod. 2017, 75, 89–100. [Google Scholar] [CrossRef]
- Finch, C.A. Wood adhesives: Chemistry and technology. Edited by A. Pizzi, Marcel Dekkar, New York and Basel, 1983. Br. Polym. J. 1984, 16, 324. [Google Scholar] [CrossRef]
- Comandini, P.; Lerma-García, M.J.; Simó-Alfonso, E.F.; Toschi, T.G. Tannin analysis of chestnut bark samples (Castanea sativa Mill.) by HPLC-DAD-MS. Food Chem. 2014, 157, 290–295. [Google Scholar] [CrossRef]
- Quideau, S.; Jourdes, M.; Lefeuvre, D.; Montaudon, D.; Saucier, C.; Glories, Y.; Pardon, P.; Pourquier, P. The chemistry of wine polyphenolic C-glycosidic ellagitannins targeting human topoisomerase II. Chem. A Eur. J. 2005, 11, 6503–6513. [Google Scholar] [CrossRef] [PubMed]
- Botondi, R.; Vailati, M.; Bellincontro, A.; Massantini, R.; Forniti, R.; Mencarelli, F. Technological Parameters of Water Curing Affect Postharvest Physiology and Storage of Marrons (Castanea Sativa Mill., Marrone Fiorentino). Postharvest Biol. Technol. 2009, 51, 97–103. [Google Scholar] [CrossRef]
- Nazzaro, M.; Barbarisi, C.; La Cara, F.; Volpe, M.G. Chemical and Biochemical Characterisation of an Igp Ecotype Chestnut Subjected to Different Treatments. Food Chem. 2011, 128, 930–936. [Google Scholar] [CrossRef]
- Blaiotta, G.; Di Capua, M.; Romano, A.; Coppola, R.; Aponte, M. Optimization of Water Curing for the Preservation of Chestnuts (Castanea Sativa Mill.) and Evaluation of Microbial Dynamics During Process. Food Microbiol. 2014, 42, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Echegaray, N.; Gómez, B.; Barba, F.J.; Franco, D.; Estévez, M.; Carballo, J.; Marszałek, K.; Lorenzo, J.M. Chestnuts and by-products as source of natural antioxidants in meat and meat products: A review. Trends Food Sci. Technol. 2018, 82, 110–121. [Google Scholar] [CrossRef]
- Pasch, H.; Pizzi, A. Considerations on the macromolecular structure of chestnut ellagitannins by matrix-assisted laser desorption/ionization-time-of-flight mass spectrometry. J. Appl. Polym. Sci. 2002, 85, 429–437. [Google Scholar] [CrossRef]
- Salminen, J.-P. Two-Dimensional Tannin Fingerprints by Liquid Chromatography Tandem Mass Spectrometry offer a new dimension to plant tannin analyses and Help to visualize the tannin diversity in plants. J. Agric. Food Chem. 2018, 66, 9162–9171. [Google Scholar] [CrossRef] [PubMed]
- Abid, M.; Yaich, H.; Cheikhrouhou, S.; Khemakhem, I.; Bouaziz, M.; Attia, H.; Ayadi, M.A. Antioxidant properties and phenolic profile characterization by LC–MS/MS of selected Tunisian pomegranate peels. J. Food Sci. Technol. 2017, 54, 2890–2901. [Google Scholar] [CrossRef] [PubMed]
- Mena, P.; Calani, L.; Dall’Asta, C.; Galaverna, G.; García-Viguera, C.; Bruni, R.; Crozier, A.; Del Rio, D. Rapid and comprehensive evaluation of (poly)phenolic compounds in pomegranate (Punica granatum L.) juice by UHPLC-MSn. Molecules 2012, 17, 14821–14840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreira, J.C.; Pereira, J.A.; Oliveira, M.B.; Ferreira, I.C. Sugars profiles of different chestnut (Castanea sativa Mill.) and almond (Prunus dulcis) cultivars by HPLC-RI. Plant. Foods Hum. Nutr. 2010, 65, 38–43. [Google Scholar] [CrossRef]
- van der Hooft, J.J.J.; Vervoort, J.; Bino, R.J.; Beekwilder, J.; de Vos, R.C.H. Polyphenol identification based on systematic and robust high-resolution accurate mass spectrometry fragmentation. Anal. Chem. 2011, 83, 409–416. [Google Scholar] [CrossRef]
- Cacciola, N.A.; Cerrato, A.; Capriotti, A.L.; Cavaliere, C.; D’Apolito, M.; Montone, C.M.; Piovesana, S.; Squillaci, G.; Peluso, G.; Laganà, A. Untargeted Characterization of Chestnut (Castanea sativa Mill.) Shell Polyphenol Extract: A Valued Bioresource for Prostate Cancer Cell Growth Inhibition. Molecules 2020, 25, 2730. [Google Scholar] [CrossRef]
- Li, H.J.; Deinzer, M.L. The Mass Spectral Analysis of Isolated Hops a-Type Proanthocyanidins by Electrospray Ionization Tandem Mass Spectrometry. J. Mass Spectrom. 2008, 43, 1353–1363. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Bajpai, V.; Kumar, S.; Sharma, K.R.; Kumar, B. Profiling of gallic and ellagic acid derivatives in different plant parts of Terminalia arjuna by HPLC-ESI-QTOF-MS/MS. Nat. Prod. Commun. 2016, 11, 239–244. [Google Scholar] [CrossRef] [Green Version]
- Bowers, J.J.; Gunawardena, H.P.; Cornu, A.; Narvekar, A.S.; Richieu, A.; Deffieux, D.; Quideau, S.; Nishanth, T. Rapid Screening of Ellagitannins in Natural Sources Via Targeted Reporter Ion Triggered Tandem Mass Spectrometry. Sci. Rep. 2018, 8, 10399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jermini, M.; Conedera, M.; Sieber, T.N.; Sassella, A.; Schärer, H.; Jelmini, G.; Höhn, E. Influence of Fruit Treatments on Perishability During Cold Storage of Sweet Chestnuts. J. Sci. Food Agric. 2006, 86, 877–885. [Google Scholar] [CrossRef]
- Migliorini, M.; Funghini, L.; Marinelli, C.; Turchetti, T.; Canuti, S.; Zanoni, B. Study of Water Curing for the Preservation of Marrons (Castanea Sativa Mill., Marrone Fiorentino Cv). Postharvest Biol. Technol. 2010, 56, 95–100. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, G.; De Pascale, S.; Aponte, M.; Scaloni, A.; Addeo, F.; Caira, S. Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants. Molecules 2021, 26, 2335. https://doi.org/10.3390/molecules26082335
Pinto G, De Pascale S, Aponte M, Scaloni A, Addeo F, Caira S. Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants. Molecules. 2021; 26(8):2335. https://doi.org/10.3390/molecules26082335
Chicago/Turabian StylePinto, Gabriella, Sabrina De Pascale, Maria Aponte, Andrea Scaloni, Francesco Addeo, and Simonetta Caira. 2021. "Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants" Molecules 26, no. 8: 2335. https://doi.org/10.3390/molecules26082335
APA StylePinto, G., De Pascale, S., Aponte, M., Scaloni, A., Addeo, F., & Caira, S. (2021). Polyphenol Profiling of Chestnut Pericarp, Integument and Curing Water Extracts to Qualify These Food By-Products as a Source of Antioxidants. Molecules, 26(8), 2335. https://doi.org/10.3390/molecules26082335