Green Synthesis of Oxoquinoline-1(2H)-Carboxamide as Antiproliferative and Antioxidant Agents: An Experimental and In-Silico Approach to High Altitude Related Disorders
Abstract
:1. Introduction
2. Results
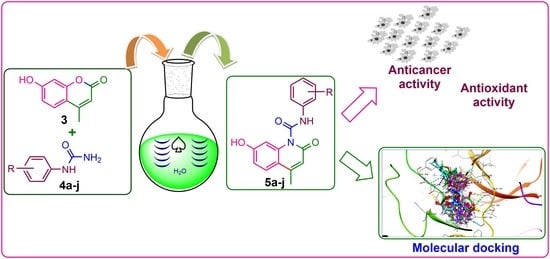
2.1. Synthesis
2.2. Optimization of Reaction Conditions
2.3. Exploration of Methodology
2.4. Antiproliferative Activity
2.5. Antioxidant Activity
2.6. Molecular Docking
2.7. ADME Studies
3. Discussion
4. Materials and Methods
4.1. Synthesis
4.2. Antiproliferative Activity
4.3. Antioxidant Activity
4.4. Molecular Docking Studies
4.5. ADME Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Nita, M.; Grzybowski, A. The Role of the Reactive Oxygen Species and Oxidative Stress in the Pathomechanism of the Age-Related Ocular Diseases and Other Pathologies of the Anterior and Posterior Eye Segments in Adults. Oxid. Med. Cell. Longev. 2016, 2016, 3164734. [Google Scholar] [CrossRef] [Green Version]
- Dosek, A.; Ohno, H.; Acs, Z.; Taylor, A.W.; Radak, Z. High altitude and oxidative stress. Respir. Physiol. Neurobiol. 2007, 158, 128–131. [Google Scholar] [CrossRef]
- Zuo, L.; Hallman, A.H.; Yousif, M.K.; Chien, M.T. Oxidative stress, respiratory muscle dysfunction, and potential therapeutics in chronic obstructive pulmonary disease. Front. Biol. 2012, 7, 506–513. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef]
- Roberts, C.K.; Sindhu, K.K. Oxidative stress and metabolic syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Sorce, S.; Krause, K.-H. NOX Enzymes in the Central Nervous System: From Signaling to Disease. Antioxid. Redox Signal. 2009, 11, 2481–2504. [Google Scholar] [CrossRef]
- Liao, D.; Corle, C.; Seagroves, T.; Johnson, R.S. Hypoxia-Inducible Factor-1α Is a Key Regulator of Metastasis in a Transgenic Model of Cancer Initiation and Progression. Cancer Res. 2007, 67, 563–572. [Google Scholar] [CrossRef] [Green Version]
- Uttara, B.; Singh, A.V.; Zamboni, P.; Mahajan, R.T. Oxidative Stress and Neurodegenerative Diseases: A Review of Upstream and Downstream Antioxidant Therapeutic Options. Curr. Neuropharmacol. 2009, 7, 65–74. [Google Scholar] [CrossRef] [Green Version]
- Frijhoff, J.; Winyard, P.G.; Zarkovic, N.; Davies, S.S.; Stocker, R.; Cheng, D.; Knight, A.R.; Taylor, E.L.; Oettrich, J.; Ruskovska, T.; et al. Clinical Relevance of Biomarkers of Oxidative Stress. Antioxid. Redox Signal. 2015, 23, 1144–1170. [Google Scholar] [CrossRef] [Green Version]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020, in press. [Google Scholar] [CrossRef]
- Boland, M.L.; Chourasia, A.H.; MacLeod, K.F. Mitochondrial Dysfunction in Cancer. Front. Oncol. 2013, 3, 292. [Google Scholar] [CrossRef] [Green Version]
- WHO Report on Cancer: Setting Priorities, Investing Wisely and Providing Care for All; World Health Organization: Geneva, Switzerland, 2020; ISBN 978-92-4-000129-9. Available online: https://apps.who.int/iris/handle/10665/330745 (accessed on 24 May 2021).
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [Green Version]
- Kharissova, O.V.; Kharisov, B.I.; González, C.M.O.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef] [Green Version]
- Menges, N. The Role of Green Solvents and Catalysts at the Future of Drug Design and of Synthesis. In Green Chemistry; Saleh, H.E.D., Koller, M., Eds.; IntechOpen: London, UK, 2018. [Google Scholar] [CrossRef] [Green Version]
- Dallinger, D.; Kappe, C.O. Microwave-Assisted Synthesis in Water as Solvent. Chem. Rev. 2007, 107, 2563–2591. [Google Scholar] [CrossRef]
- Zang, H.; Zhang, Y.; Zang, Y.; Cheng, B.-W. An efficient ultrasound-promoted method for the one-pot synthesis of 7,10,11,12-tetrahydrobenzo[c]acridin-8(9H)-one derivatives. Ultrason. Sonochem. 2010, 17, 495–499. [Google Scholar] [CrossRef]
- Jarag, K.; Pinjari, D.V.; Pandit, A.B.; Shankarling, G. Synthesis of chalcone (3-(4-fluorophenyl)-1-(4-methoxyphenyl)prop-2-en-1-one): Advantage of sonochemical method over conventional method. Ultrason. Sonochem. 2011, 18, 617–623. [Google Scholar] [CrossRef]
- Bakht, M.A.; Ansari, M.J.; Riadi, Y.; Ajmal, N.; Ahsan, M.J.; Yar, M.S. Physicochemical characterization of benzalkonium chloride and urea based deep eutectic solvent (DES): A novel catalyst for the efficient synthesis of isoxazolines under ultrasonic irradiation. J. Mol. Liq. 2016, 224, 1249–1255. [Google Scholar] [CrossRef]
- Mason, T.J. Sonochemistry and the environment—Providing a “green” link between chemistry, physics and engineering. Ultrason. Sonochem. 2007, 14, 476–483. [Google Scholar] [CrossRef]
- Yadav, J.; Reddy, B.; Reddy, K.S. Ultrasound-accelerated synthesis of chiral allylic alcohols promoted by indium metal. Tetrahedron 2003, 59, 5333–5336. [Google Scholar] [CrossRef]
- Geesi, M.H.; Moustapha, M.E.; Bakht, M.A.; Riadi, Y. Ultrasound-accelerated green synthesis of new quinolin-2-thione derivatives and antimicrobial evaluation against Escherichia coli and Staphylococcus aureus. Sustain. Chem. Pharm. 2020, 15, 100195. [Google Scholar] [CrossRef]
- Geesi, M.H.; Ouerghi, O.; Elsanousi, A.; Kaiba, A.; Riadi, Y. Ultrasound-Assisted Preparation of Cu-Doped TiO2 Nanoparticles as a Nanocatalyst for Sonochemical Synthesis of Pyridopyrimidines. Polycycl. Aromat. Compd. 2020, 42, 80–90. [Google Scholar] [CrossRef]
- Rao, M.S.; Haritha, M.; Chandrasekhar, N.; Rao, M.V.B.; Pal, M. Ultrasound mediated synthesis of 6-substituted 2,3-dihydro-1H-pyrrolo[3,2,1-ij]quinoline derivatives and their pharmacological evaluation. Arab. J. Chem. 2019, 12, 2697–2703. [Google Scholar] [CrossRef] [Green Version]
- Dofe, V.S.; Sarkate, A.P.; Shaikh, Z.M.; Gill, C.H. Ultrasound-Mediated Synthesis of Novel 1,2,3-Triazole-Based Pyrazole and Pyrimidine Derivatives as Antimicrobial Agents. J. Heterocycl. Chem. 2017, 54, 3195–3201. [Google Scholar] [CrossRef]
- Tabassum, S.; Govindaraju, S.; Khan, R.R.; Pasha, M.A. Ultrasound mediated, green innovation for the synthesis of polysub-stituted 1,4-dihydropyridines. RSC Adv. 2016, 6, 29802–29810. [Google Scholar] [CrossRef] [Green Version]
- Castro-Puyana, M.; Marina, M.L.; Plaza, M. Water as green extraction solvent: Principles and reasons for its use. Curr. Opin. Green Sustain. Chem. 2017, 5, 31–36. [Google Scholar] [CrossRef]
- Simon, M.-O.; Li, C.-J. Green chemistry oriented organic synthesis in water. Chem. Soc. Rev. 2012, 41, 1415–1427. [Google Scholar] [CrossRef]
- Lee, S.Y.; Cheon, C.-H. On-Water Synthesis of 2-Substituted Quinolines from 2-Aminochalcones Using Benzylamine as the Nucleophilic Catalyst. J. Org. Chem. 2018, 83, 13036–13044. [Google Scholar] [CrossRef]
- Gopi, P.; Sarveswari, S. Effective water mediated green synthesis of polysubstituted quinolines without energy expenditure. Mon. Chem. Chem. Mon. 2017, 148, 1043–1049. [Google Scholar] [CrossRef]
- Borah, G.; Borah, P.; Bhuyan, A.; Banik, B.K. Facile Synthesis of Quinolines in Water. Curr. Org. Chem. 2021, 25, 175–208. [Google Scholar] [CrossRef]
- Afzal, O.; Kumar, S.; Haider, R.; Ali, R.; Kumar, R.; Jaggi, M.; Bawa, S. A review on anticancer potential of bioactive heterocycle quinoline. Eur. J. Med. Chem. 2015, 97, 871–910. [Google Scholar] [CrossRef]
- Wissner, A.; Overbeek, E.; Reich, M.F.; Floyd, M.B.; Johnson, B.D.; Mamuya, N.; Rosfjord, E.C.; Discafani, C.; Davis, R.; Shi, X. Synthesis and structure-activity relationships of 6,7-disubstituted 4-anilinoquinoline-3-carbonitriles. The design of an orally active, irreversible inhibitor of thetyrosine kinase activity of the epidermal growth factor receptor (EGFR) and the human ep-idermal growth factor receptor-2 (HER-2). J. Med. Chem. 2003, 46, 49–63. [Google Scholar]
- Cherian, M.A.; Ma, C.X. The role of neratinib in HER2-driven breast cancer. Future Oncol. 2017, 13, 1931–1943. [Google Scholar] [CrossRef]
- Genis, C.; Sippel, K.H.; Case, N.; Cao, W.; Avvaru, B.S.; Tartaglia, L.J.; Govindasamy, L.; Tu, C.; Agbandje-McKenna, M.; Silverman, D.N.; et al. Design of a Carbonic Anhydrase IX Active-Site Mimic to Screen Inhibitors for Possible Anticancer Properties. Biochemistry 2009, 48, 1322–1331. [Google Scholar] [CrossRef] [Green Version]
- Supuran, C.T.; Briganti, F.; Tilli, S.; Chegwidden, W.R.; Scozzafava, A. Carbonic anhydrase inhibitors: Sulfonamides as anti-tumor agents? Bioorg. Med. Chem. 2001, 9, 703–714. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Bakht, M.A.; Ahsan, M.J. Ultrasound promoted synthesis of N-(substituted phenyl)-2-(7-hydroxy-4-methyl-2H-chromen-2-ylidene)hydrazine-1-carboxamides as cytotoxic and antioxidant agents. J. Mol. Struct. 2021, 1238, 130452. [Google Scholar] [CrossRef]
- Prousis, K.C.; Avlonitis, N.; Heropoulos, G.A.; Calogeropoulou, T. FeCl3-catalysed ultrasonic-assisted, solvent-free synthesis of 4-substituted coumarins. A useful complement to the Pechmann reaction. Ultrason. Sonochem. 2014, 21, 937–942. [Google Scholar] [CrossRef]
- Ahsan, M.J.; Yadav, R.P.; Saini, S.; Hassan, M.Z.; Bakht, M.A.; Jadav, S.S.; Al-Tamimi, A.B.S.; Geesi, M.H.; Ansari, Y.; Khalilullah, H.; et al. Synthesis, Cytotoxic Evaluation, and Molecular Docking Studies of New Oxadiazole Analogues. Lett. Org. Chem. 2017, 15, 49–56. [Google Scholar] [CrossRef]
- Bakht, M.A.; Azam, F.; Ali, A.; Thomas, R.; Pooventhiran, T.; Ali, A.; Ahsan, M.J. Synthesis and biological studies of oxoquinolines: Experimental and theoretical investigations. J. Mol. Struct. 2022, 1248, 131509. [Google Scholar] [CrossRef]
- Ali, A.; Ali, A.; Salahuddin; Bakht, M.A.; Ahsan, M.J. Synthesis and Biological Evaluations of N-(4-Substituted Phenyl)-7-Hydroxy-4-Methyl-2-Oxoquinoline-1(2H)-Carbothioamides. Polycycl. Aromat. Compd. 2021, 1–12. [Google Scholar] [CrossRef]
- National Cancer Institute (NCI). DTP Homepage: Cancer Screen 09/2014: NCI-60 DTP Human Tumor Cell Line Screen. The National Cancer Institute (NCI) Is Gratefully Acknowledged for Its Excellent Screening Service. Available online: http://dtp.nci.nih.gov (accessed on 9 May 2021).
- Monks, A.; Scudiero, D.; Skehan, P.; Shoemaker, R.; Paull, K.; Vistica, D.; Hose, C.; Langley, J.; Cronise, P.; Vaigro-Wolff, A.; et al. Feasibility of a High-Flux Anticancer Drug Screen Using a Diverse Panel of Cultured Human Tumor Cell Lines. JNCI J. Natl. Cancer Inst. 1991, 83, 757–766. [Google Scholar] [CrossRef]
- Boyd, M.R.; Paull, K.D. Some practical considerations and applications of the National Cancer Institute in vitro anticancer drug discovery screen. Drug Dev. Res. 1995, 34, 91–109. [Google Scholar] [CrossRef]
- Shoemaker, R.H. The NCI60 human tumour cell line anticancer drug screen. Nat. Rev. Cancer 2006, 6, 813–823. [Google Scholar] [CrossRef]
- Koleva, I.I.; Van Beek, T.A.; Linssen, J.P.H.; De Groot, A.; Evstatieva, L.N. Screening of Plant Extracts for Antioxidant Activity: A Comparative Study on Three Testing Methods. Phytochem. Anal. 2002, 13, 8–17. [Google Scholar] [CrossRef]
- PDB of EGFR. Available online: https://www.rcsb.org/structure/3W2R (accessed on 24 May 2021).
- Sogabe, S.; Kawakita, Y.; Igaki, S.; Iwata, H.; Miki, H.; Cary, D.R.; Takagi, T.; Takagi, S.; Ohta, Y.; Ishikawa, T. Structure-Based Approach for the Discovery of Pyrrolo[3,2-d]pyrimidine-Based EGFR T790M/L858R Mutant Inhibitors. ACS Med. Chem. Lett. 2012, 4, 201–205. [Google Scholar] [CrossRef] [Green Version]
- PDB of CA. Available online: https://www.rcsb.org/structure/3DC3 (accessed on 16 October 2021).
- SwissADME. Available online: http://www.swissadme.ch/ (accessed on 27 March 2021).
- Gundersen, L.-L.; Nissen-Meyer, J.; Spilsberg, B. Synthesis and Antimycobacterial Activity of 6-Arylpurines: The Requirements for the N-9 Substituent in Active Antimycobacterial Purines. J. Med. Chem. 2002, 45, 1383–1386. [Google Scholar] [CrossRef]
- Heifets, L.B.; Flory, M.A.; Lindholm-Levy, P.J. Does pyrazinoic acid as an active moiety of pyrazinamide have specific activity against Mycobacterium tuberculosis? Antimicrob. Agents Chemother. 1989, 33, 1252–1254. [Google Scholar] [CrossRef] [Green Version]
- Refsgaard, H.H.F.; Jensen, B.F.; Brockhoff, P.B.; Padkjær, S.B.; Guldbrandt, M.; Christensen, M.S. In Silico Prediction of Membrane Permeability from Calculated Molecular Parameters. J. Med. Chem. 2005, 48, 805–811. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.-Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular Properties That Influence the Oral Bioavailability of Drug Candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Baell, J.B.; Holloway, G.A. New Substructure Filters for Removal of Pan Assay Interference Compounds (PAINS) from Screening Libraries and for Their Exclusion in Bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef] [Green Version]
- Bakonyi, T.; Radak, Z. High Altitude and Free Radicals. J. Sports Sci. Med. 2004, 3, 64–69. [Google Scholar]
- Askew, E. Work at high altitude and oxidative stress: Antioxidant nutrients. Toxicology 2002, 180, 107–119. [Google Scholar] [CrossRef]
- Forwand, S.A.; Landowne, M.; Follansbee, J.N.; Hansen, J.E. Effect of Acetazolamide on Acute Mountain Sickness. N. Engl. J. Med. 1968, 279, 839–845. [Google Scholar] [CrossRef]
- Shekhar, S.; Liu, Y.; Wang, S.; Zhang, H.; Fang, X.; Zhang, J.; Fan, L.; Zheng, B.; Roman, R.J.; Wang, Z.; et al. Novel Mechanistic Insights and Potential Therapeutic Impact of TRPC6 in Neurovascular Coupling and Ischemic Stroke. Int. J. Mol. Sci. 2021, 22, 2074. [Google Scholar] [CrossRef]









 | ||||
|---|---|---|---|---|
| Entry | Reflux/)))))/Fused | Solvent | Reaction Time | Yield b (%) |
| 1 | ))))) | Ethylacetate | 20 min | 34 |
| 2 | ))))) | Methanol | 20 min | 60 |
| 3 | ))))) | Ethanol | 20 min | 67 |
| 4 | ))))) | Toluene | 20 min | 44 |
| 5 | ))))) | Dioxane | 20 min | 36 |
| 6 | ))))) | Acetonitrile | 20 min | 54 |
| 7 | ))))) | Dichloromethane | 20 min | 59 |
| 8 | ))))) | Dimethyl sulfoxide | 20 min | 49 |
| 9 | ))))) | Glacial acetic acid | 20 min | 65 |
| 10 | Fused at 200 °C | Solvent free | 60 min | 71 |
| 11 | Reflux | Water | 240 min | 82 |
| 12 | ))))) | Water | 20 min | 91 |
 | ||||||
|---|---|---|---|---|---|---|
| S. No. | Compound | R | Mp (ºC) | Rf * | Yield a (Time in min) | |
| Fused at 200 °C b | )))))) c | |||||
| 1 | 5a | H | 78–80 | 0.66 | 82% (60 min) | 91% (20 min) |
| 2 | 5b | 4-Cl | 160–162 | 0.62 | 72% (60 min) | 90% (20 min) |
| 3 | 5c | 4-CH3 | 140–142 | 0.71 | 60% (60 min) | 81% (5 min) |
| 4 | 5d | 4-OCH3 | 128–130 | 0.69 | 58% (60 min) | 80% (20 min) |
| 5 | 5e | 4-CF3 | 142–144 | 0.63 | 71% (60 min) | 90% (20 min) |
| 6 | 5f | 2-Cl | 152–154 | 0.68 | 62% (60 min) | 91% (20 min) |
| 7 | 5g | 2-CH3 | 102–104 | 0.72 | 68% (60 min) | 78% (20 min) |
| 8 | 5h | 2-OCH3 | 110–112 | 0.78 | 69% (60 min) | 80% (20 min) |
| 9 | 5i | 2-OH-5-Cl | 156–158 | 0.74 | 56% (60 min) | 70% (25 min) |
| 10 | 5j | 3-Cl-4-F | 180–182 | 0.64 | 84% (60 min) | 92% (20 min) |
| Compound/NSC Code | Cancer Cell Lines Assay in Single Dose Assay 10 µM Concentration | ||
|---|---|---|---|
| The Most Sensitive Cell Lines | GP | %GI | |
| 5a NSC 805519 | TK-10 (Renal Cancer) | 17.10 | 82.90 |
| UO-31 (Renal Cancer) | 76.90 | 23.10 | |
| HOP-92 (Non Small Cell Lung Cancer) | 84.00 | 16.00 | |
| CAKI 1 (Renal Cancer) | 87.61 | 12.39 | |
| HS 578T (Breast Cancer) | 88.50 | 11.50 | |
| 5b NSC 805508 | HCT116 (Colon Cancer) | 87.36 | 12.64 |
| UO-31 (Renal Cancer) | 87.82 | 12.18 | |
| K562 (Leukemia) | 90.23 | 9.77 | |
| SNB-75 (CNS Cancer) | 91.49 | 8.51 | |
| NCI-H23 (Non-Small Cell Lung Cancer) | 92.81 | 7.19 | |
| 5c NSC 805512 | UO-31 (Renal Cancer) | 82.04 | 17.96 |
| HOP-92 (Non Small Cell Lung Cancer) | 85.60 | 14.40 | |
| OVCAR-5 (Ovarian Cancer) | 87.13 | 12.87 | |
| SF-539 (CNS Cancer) | 89.28 | 10.72 | |
| MALME-3M (Melanoma) | 90.95 | 9.05 | |
| 5d NSC 805516 | HOP-92 (Non Small Cell Lung Cancer) | 77.53 | 22.47 |
| UO-31 (Renal Cancer) | 74.56 | 25.44 | |
| SNB-75 (CNS Cancer) | 86.10 | 13.90 | |
| MDA-MB-231/ATCC (Breast Cancer) | 86.25 | 13.75 | |
| NCI-H23 (Non-Small Cell Lung Cancer) | 87.17 | 12.83 | |
| 5e NSC 805511 | A498 (Renal Cancer) | 81.52 | 18.48 |
| UO-31 (Renal Cancer) | 87.76 | 12.24 | |
| CAKI-1 (Renal Cancer) | 89.97 | 10.03 | |
| SNB-75 (CNS Cancer) | 93.09 | 6.91 | |
| UACC-62 (Melanoma) | 94.90 | 5.10 | |
| 5f NSC 805517 | UO-31 (Renal Cancer) | 76.87 | 23.13 |
| HOP-92 (Non-Small Cell Lung Cancer) | 79.74 | 20.26 | |
| NCI-H322M (Non Small Cell Lung Cancer) | 88.75 | 10.79 | |
| HOP-62 (Non-Small Cell Lung Cancer) | 89.21 | 7.79 | |
| CAKI-1 (Renal Cancer) | 92.27 | 7.73 | |
| 5g NSC 805513 | UO-31 (Renal Cancer) | 80.06 | 19.94 |
| HOP-92 (Non-Small Cell Lung Cancer) | 89.43 | 10.57 | |
| MDA-MB-231/ATCC (Breast Cancer) | 90.02 | 9.98 | |
| CAKI-1 (Renal Cancer) | 92.87 | 7.13 | |
| HS 578T (Breast Cancer) | 94.23 | 5.77 | |
| 5h NSC 805515 | UO-31 (Renal Cancer) | 88.62 | 11.38 |
| HOP-92 (Non-Small Cell Lung Cancer) | 88.90 | 11.10 | |
| NCI-H226 (Non-Small Cell Lung Cancer) | 93.46 | 6.54 | |
| RXF 393 (Renal Cancer) | 94.83 | 5.17 | |
| HCC-2998 (Colon Cancer) | 95.92 | 4.08 | |
| 5i NSC 805520 | HOP-92 (Non-Small Cell Lung Cancer) | 72.79 | 27.21 |
| UO-31 (Renal Cancer) | 79.09 | 20.91 | |
| CCRF-CEM (Leukemia) | 80.64 | 19.36 | |
| MALME-3M (Melanoma) | 87.21 | 12.79 | |
| NCI-H322M (Non-Small Cell Lung Cancer) | 88.16 | 11.84 | |
| 5j NSC 805518 | CCRF-CEM (Leukemia) | 41.39 | 58.61 |
| UO-31 (Renal Cancer) | 70.00 | 30.00 | |
| HOP-92 (Non Small Cell Lung Cancer) | 72.56 | 27.44 | |
| A498 (Renal Cancer) | 79.47 | 20.53 | |
| HOP-62 (Non Small Cell Lung Cancer) | 82.44 | 17.56 | |
| Imatinib * NSC 759854 | HT29 (Colon Cancer) | 52.9 | 47.1 |
| HOP-92 (Non-Small Cell Lung Cancer) | 56.3 | 43.7 | |
| MDA-MB-468 (Breast Cancer) | 70.9 | 29.1 | |
| SF-539 (CNS Cancer) | 75.5 | 24.5 | |
| SK-MEL-5 (Melanoma) | 77.7 | 22.3 | |
| S. No. | Compound | Free Radical Scavenging Activity IC50 (μM) |
|---|---|---|
| 1 | 5a | 14.16 ± 0.42 |
| 2 | 5b | 24.18 ± 0.41 |
| 3 | 5d | 22.90 ± 0.89 |
| 4 | 5f | 36.32 ± 0.93 |
| 5 | 5g | 54.45 ± 0.95 |
| 6 | 5h | 24.52 ± 0.72 |
| 7 | 5j | 31.11 ± 0.91 |
| 8 | Ascorbic acid | 13.99 ± 0.89 |
| S. No. | Ligand | Docking Score | Types of Interaction |
|---|---|---|---|
| 1 | 5a | −8.839 | H-bond (Met793), π-π-Stacking (Leu788 and Ala743) |
| 2 | 5b | −9.013 | H-bond (Met793), π-π-Stacking (Met793) |
| 3 | 5c | −7.944 | H-bond (Asp855), π-π-Stacking (Thr854 and Phe856) |
| 4 | 5d | −8.216 | H-bond (Gln791), π-π-Stacking (Gln791 and Asp855) |
| 5 | 5e | −9.378 | H-bond (Met793), π-π-Stacking (Met793) |
| 6 | 5f | −8.797 | H-bond (Gln791), Halogen bond (Ala743), π-π-Stacking (Gln791) |
| 7 | 5g | −8.714 | H-bond (Asp855 and Thr854) |
| 8 | 5h | −9.416 | H-bond (Met793, Gln791 and Thr854) |
| 9 | 5i | −9.013 | H-bond (Asp855, Ala743 and Thr854) |
| 10 | 5j | −8.305 | H-bond (Asp855 and Thr854), Halogen bond (Asp855), π-π-Stacking (Leu788) |
| Parameters | Compounds | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5a | 5b | 5c | 5d | 5e | 5f | 5g | 5h | 5i | 5j | |
| No. H-bond acceptor | 3 | 3 | 3 | 4 | 6 | 3 | 3 | 4 | 4 | 4 |
| No. H-bond donor | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 2 | 3 | 2 |
| LogPO/W(iLOGP) | 1.82 | 2.39 | 2.02 | 2.40 | 2.58 | 2.22 | 2.13 | 2.15 | 2.00 | 2.62 |
| No. rotatable bonds | 3 | 3 | 3 | 4 | 4 | 3 | 3 | 4 | 3 | 3 |
| TPSA | 71.33 | 71.33 | 71.33 | 80.56 | 71.33 | 71.33 | 71.33 | 80.56 | 91.56 | 71.33 |
| Log KP (skin permeation) | −6.50 | −6.27 | −6.33 | −6.70 | −6.29 | −6.27 | −6.33 | −6.70 | −6.61 | −6.31 |
| Lipinski’s rule violation | No | No | No | No | No | No | No | No | No | No |
| Bioavailability score | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 | 0.55 |
| GI absorption | High | High | High | High | High | High | High | High | High | High |
| BBB permeation | Yes | Yes | Yes | No | No | Yes | Yes | No | No | Yes |
| PAINS alerts | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| P-pg substrate | No | No | No | No | No | No | No | No | No | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Ali, A.; Warsi, M.H.; Rahman, M.A.; Ahsan, M.J.; Azam, F. Green Synthesis of Oxoquinoline-1(2H)-Carboxamide as Antiproliferative and Antioxidant Agents: An Experimental and In-Silico Approach to High Altitude Related Disorders. Molecules 2022, 27, 309. https://doi.org/10.3390/molecules27010309
Ali A, Ali A, Warsi MH, Rahman MA, Ahsan MJ, Azam F. Green Synthesis of Oxoquinoline-1(2H)-Carboxamide as Antiproliferative and Antioxidant Agents: An Experimental and In-Silico Approach to High Altitude Related Disorders. Molecules. 2022; 27(1):309. https://doi.org/10.3390/molecules27010309
Chicago/Turabian StyleAli, Amena, Abuzer Ali, Musarrat Husain Warsi, Mohammad Akhlaquer Rahman, Mohamed Jawed Ahsan, and Faizul Azam. 2022. "Green Synthesis of Oxoquinoline-1(2H)-Carboxamide as Antiproliferative and Antioxidant Agents: An Experimental and In-Silico Approach to High Altitude Related Disorders" Molecules 27, no. 1: 309. https://doi.org/10.3390/molecules27010309
APA StyleAli, A., Ali, A., Warsi, M. H., Rahman, M. A., Ahsan, M. J., & Azam, F. (2022). Green Synthesis of Oxoquinoline-1(2H)-Carboxamide as Antiproliferative and Antioxidant Agents: An Experimental and In-Silico Approach to High Altitude Related Disorders. Molecules, 27(1), 309. https://doi.org/10.3390/molecules27010309