Anion Binding by Fluorescent Ureido-Hexahomotrioxacalix[3]arene Receptors: An NMR, Absorption and Emission Spectroscopic Study
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Conformational Analysis
2.2. Anion and Ion-Pair Recognition
2.2.1. Proton NMR Studies
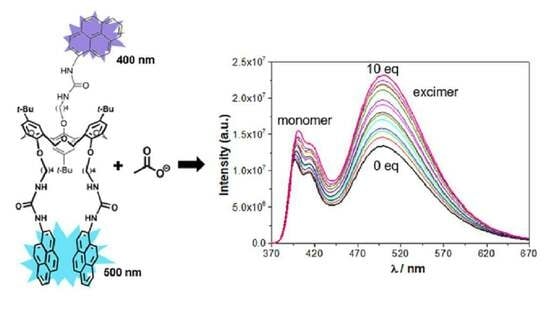
2.2.2. UV–Vis Absorption and Fluorescence Studies

2.2.3. Temperature Dependence of the Association Constants—Thermodynamic Analysis and Simulation
3. Experiments
3.1. General Information
3.2. Procedure for the Synthesis of Ureas 4a and 4c, and Thiourea 4b
3.3. 1H NMR Titrations
3.4. UV–Vis Absorption and Fluorescence Studies
3.5. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kim, J.S.; Quang, D.T. Calixarene-derived fluorescent probes. Chem. Rev. 2007, 107, 3780–3799. [Google Scholar] [CrossRef]
- Kumar, R.; Jung, Y.; Kim, J.S. Fluorescent calixarene hosts. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Urdorf, Switzerland, 2016; pp. 743–760. [Google Scholar]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting fluorescent calixarenes: From molecular sensors to smart materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Calixarenes, An Introduction; Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Neri, P.; Sessler, J.L.; Wang, M.-X. (Eds.) Calixarenes and Beyond; Springer International Publishing: Urdorf, Switzerland, 2016. [Google Scholar]
- Jeon, N.J.; Ryu, B.J.; Lee, B.H.; Nam, K.C. Fluorescent sensing of tetrahedral anions with a pyrene urea derivative of calix[4]arene chemosensor. Bull. Korean Chem. Soc. 2009, 30, 1675–1677. [Google Scholar]
- Hung, H.C.; Chang, Y.Y.; Luo, L.; Hung, C.H.; Diau, E.W.G.; Chung, W.S. Different sensing modes of fluoride and acetate based on a calix[4]arene with 25,27-bistriazolylmethylpyrenylacetamides. Photochem. Photobiol. Sci. 2014, 13, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Sutariya, P.G.; Pandya, A.; Lodha, A.; Menon, S.K. A pyrenyl linked calix[4]arene fluorescence probe for recognition of ferric and phosphate ions. RSC Adv. 2014, 4, 34922–34926. [Google Scholar] [CrossRef]
- Chawla, H.M.; Munjal, P. Evaluation of calix[4]arene tethered Schiff bases for anion recognition. J. Lumin. 2016, 179, 114–121. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseinzadeh, R.; Zadmard, R.; Mohadjerani, M. Highly selective colorimetric and fluorescent chemosensor for fluoride based on fluorenone armed calix[4]arene. Sens. Actuators B 2017, 241, 690–697. [Google Scholar] [CrossRef]
- Uttam, B.; Kandi, R.; Hussain, M.A.; Rao, C.P. Fluorescent lower rim 1,3-dibenzooxadiazole conjugate of calix[4]arene in selective sensing of fluoride in solution and in biological cells using confocal microscopy. J. Org. Chem. 2018, 83, 11850–11859. [Google Scholar] [CrossRef]
- Li, Z.Y.; Su, H.K.; Tong, H.X.; Yin, Y.; Xiao, T.; Sun, X.Q.; Jiang, J.; Wang, L. Calix[4]arene containing thiourea and coumarin functionality as highly selective fluorescent and colorimetric chemosensor for fluoride ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 200, 307–312. [Google Scholar] [CrossRef]
- Nemati, M.; Hosseinzadeh, R.; Mohadjerani, M. Colorimetric and fluorimetric chemosensor based on upper rim-functionalized calix[4]arene for selective detection of fluoride ion. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 245, 118950. [Google Scholar] [CrossRef]
- Capici, C.; De Zorzi, R.; Gargiulli, C.; Gattuso, G.; Geremia, S.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Puntoriero, F. Calix[5]crown-3-based heteroditopic receptors for n-butylammonium halides. Tetrahedron 2010, 66, 4987–4993. [Google Scholar] [CrossRef]
- Jeon, N.J.; Ryu, B.J.; Park, K.D.; Lee, Y.J.; Nam, K.C. Tetrahedral anions selective fluorescent calix[6]arene receptor containing urea and pyrene moieties. Bull. Korean Chem. Soc. 2010, 31, 3809–3811. [Google Scholar] [CrossRef]
- Brunetti, E.; Picron, J.-F.; Flidrova, K.; Bruylants, G.; Bartik, K.; Jabin, I. Fluorescent chemosensors for anions and contact ion pairs with a cavity-based selectivity. J. Org. Chem. 2014, 79, 6179–6188. [Google Scholar] [CrossRef] [PubMed]
- Evans, N.H.; Beer, P.D. Advances in anion supramolecular chemistry: From recognition to chemical applications. Angew. Chem. Int. Ed. 2014, 53, 11716–11754. [Google Scholar] [CrossRef] [PubMed]
- Busschaert, N.; Caltagirone, C.; Van Rossom, W.; Gale, P.A. Applications of supramolecular anion recognition. Chem. Rev. 2015, 115, 8038–8155. [Google Scholar] [CrossRef]
- Gale, P.A.; Howe, E.N.W.; Wu, X. Anion receptor chemistry. Chem 2016, 1, 351–422. [Google Scholar] [CrossRef]
- He, Q.; Vargas-Zúñiga, G.I.; Kim, S.H.; Kim, S.K.; Sessler, J.L. Macrocycles as ion pair receptors. Chem. Rev. 2019, 119, 9753–9835. [Google Scholar] [CrossRef]
- Cottet, K.; Marcos, P.M.; Cragg, P.J. Fifty years of oxacalix[3]arenes: A review. Beilstein J. Org. Chem. 2012, 8, 201–226. [Google Scholar] [CrossRef]
- Marcos, P.M. Functionalization and properties of homooxacalixarenes. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Urdorf, Switzerland, 2016; pp. 445–466. [Google Scholar]
- Teixeira, F.A.; Marcos, P.M.; Ascenso, J.R.; Brancatelli, G.; Hickey, N.; Geremia, S. Selective binding of spherical and linear anions by tetraphenyl(thio)urea-based dihomooxacalix[4]arene receptors. J. Org. Chem. 2017, 82, 11383–11390. [Google Scholar] [CrossRef]
- Augusto, A.S.; Miranda, A.S.; Ascenso, J.R.; Miranda, M.Q.; Félix, V.; Brancatelli, G.; Hickey, N.; Geremia, S.; Marcos, P.M. Anion recognition by partial cone dihomooxacalix[4]arene-based receptors bearing urea groups: Remarkable affinity for benzoate ion. Eur. J. Org. Chem. 2018, 2018, 5657–5667. [Google Scholar] [CrossRef]
- Miranda, A.S.; Serbetci, D.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Hickey, N.; Geremia, S. Ditopic receptors based on dihomooxacalix[4]arenes bearing phenylurea moieties with electron-withdrawing groups for anions and organic ion pairs. Front. Chem. 2019, 7, 758. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.S.; Martelo, L.M.; Fedorov, A.A.; Berberan-Santos, M.N.; Marcos, P.M. Fluorescence properties of p-tert-butyldihomooxacalix[4]arene derivatives and the effect of anion complexation. N. J. Chem. 2017, 41, 5967–5973. [Google Scholar] [CrossRef]
- Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Schurhammer, R.; Hickey, N.; Geremia, S. Dihomooxacalix[4]arene-based fluorescent receptors for anion and organic ion pair recognition. Molecules 2020, 25, 4708. [Google Scholar] [CrossRef] [PubMed]
- Ni, X.; Zheng, X.; Redshaw, C.; Yamato, T. Ratiometric fluorescent receptors for both Zn2+ and H2PO4− ions based on a pyrenyl-linked triazole-modified homooxacalix[3]arene: A potential molecular traffic signal with an R-S latch logic circuit. J. Org. Chem. 2011, 76, 5696–5702. [Google Scholar] [CrossRef]
- Wu, Y.; Ni, X.-L.; Mou, L.; Jin, C.-C.; Redshaw, C.; Yamato, T. Synthesis of a ditopic homooxacalix[3]arene for fluorescence enhanced detection of heavy and transition metal ions. Supramol. Chem. 2015, 27, 501–507. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, J.-L.; Jiang, X.-K.; Wang, C.-Z.; Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. A novel fluorescence “on-off-on” chemosensor for Hg2+ via a water-assistant blocking heavy atom effect. Dalton Trans. 2016, 45, 14948–14953. [Google Scholar] [CrossRef]
- Wu, C.; Wang, C.-Z.; Zhu, Q.; Zeng, X.; Redshaw, C.; Yamato, T. Click synthesis of a quinoline-functionalized hexahomotrioxacalix[3]arene: A turn-on fluorescence chemosensor for Fe3+. Sens. Actuators B 2018, 254, 52–58. [Google Scholar] [CrossRef]
- Teixeira, F.A.; Ascenso, J.R.; Cragg, P.J.; Hickey, N.; Geremia, S.; Marcos, P.M. Recognition of anions, monoamine neurotransmitter and trace amine hydrochlorides by ureido-hexahomotrioxacalix[3]arene ditopic receptors. Eur. J. Org. Chem. 2020, 13, 1930–1940. [Google Scholar] [CrossRef]
- Lambert, S.; Bartik, K.; Jabin, I. Specific binding of primary ammonium ions and lysine-containing peptides in protic solvents by hexahomotrioxacalix[3]arenes. J. Org. Chem. 2020, 85, 10062–10071. [Google Scholar] [CrossRef]
- Hynes, M.J. EQNMR: A computer program for the calculation of stability constants from nuclear magnetic resonance chemical shift data. J. Chem. Soc. Dalton Trans. 1993, 311–312. [Google Scholar] [CrossRef]
- Bryantsev, V.S.; Hay, B.P. Conformational preferences and internal rotation in alkyl- and phenyl-substituted thiourea derivatives. J. Phys. Chem. A 2006, 110, 4678–4688. [Google Scholar] [CrossRef] [PubMed]
- Marcos, P.M.; Ascenso, J.R.; Segurado, M.A.P.; Cragg, P.J.; Michel, S.; Hubscher-Bruder, V.; Arnaud-Neu, F. Lanthanide cation binding properties of homooxacalixarene diethylamide derivatives. Supramol. Chem. 2011, 23, 93–101. [Google Scholar] [CrossRef]
- Appel, E.A.; Forster, R.A.; Koutsioubas, A.; Toprakcioglu, C.; Scherman, O.A. Activation energies control the macroscopic properties of physically cross-linked materials. Angew. Chem. Int. Ed. 2014, 53, 10038–10043. [Google Scholar] [CrossRef] [PubMed]
- Menezes, F.; Fedorov, A.; Baleizao, C.; Valeur, B.; Berberan-Santos, M.N. Methods for the analysis of complex fluorescence decays: Sum of Becquerel functions versus sum of exponentials. Methods Appl. Fluoresc. 2013, 1, 015002. [Google Scholar] [CrossRef]
- Gans, P.; Sabatini, A.; Vacca, A. Investigation of equilibria in solution. Determination of equilibrium constants with the HYPERQUAD suite of programs. Talanta 1996, 43, 1739–1753. [Google Scholar] [CrossRef]
- Menezes, F.; Popowicz, G.M. ULYSSES: An efficient and easy to use semi-empirical library for C++. 2022; Submitted Manuscript. [Google Scholar]
- Bannwarth, C.; Ehlert, S.; Grimme, S. GFN2-xTB—An accurate and broadly parametrized self-consistent tight-binding quantum chemical method with multipole electrostatics and density-dependent dispersion contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]
- Ehlert, S.; Stahn, M.; Spicher, S.; Grimme, S. Robust and efficient implicit solvation model for fast semiempirical methods. J. Chem. Theory Comput. 2021, 17, 4250–4261. [Google Scholar] [CrossRef]
- Avogadro: An Open-Source Molecular Builder and Visualization Tool. Version 1.20. Available online: http://avogadro.cc/ (accessed on 10 April 2022).
- Hanwell, M.D.; Curtis, D.E.; Lonie, D.C.; Vandermeersch, T.; Zurek, E.; Hutchison, G.R. Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. J. Cheminform. 2012, 4, 17. [Google Scholar] [CrossRef]
- Lindh, R.; Bernhardsson, A.; Karlström, G.; Malmqvist, P.-A. On the use of a Hessian model function in molecular geometry optimizations. Chem. Phys. Lett. 1995, 241, 423–428. [Google Scholar] [CrossRef]
- Grimme, S. Supramolecular binding thermodynamics by dispersion-corrected density functional theory. Chem. Eur. J. 2012, 18, 9955–9964. [Google Scholar] [CrossRef]
- Pracht, P.; Bohle, F.; Grimme, S. Automated exploration of the low-energy chemical space with fast quantum chemical methods. Phys. Chem. Chem. Phys. 2020, 14, 7169–7192. [Google Scholar] [CrossRef] [PubMed]
- Spicher, S.; Grimme, S. Robust atomistic modeling of materials, organometallic, and biochemical systems. Angew. Chem. 2020, 59, 15665. [Google Scholar] [CrossRef] [PubMed]
- Sure, R.; Grimme, S. Comprehensive benchmark of association (free) energies of realistic host–guest complexes. J. Chem. Theory Comput. 2015, 11, 3785–3801. [Google Scholar] [CrossRef] [PubMed]
- Hunter, J.D. Matplotlib: A 2D graphics environment. Comput. Sci. Eng. 2007, 9, 90–95. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of parameters for semiempirical methods V: Modification of NDDO approximations and application to 70 elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Korth, M. Third-generation hydrogen-bonding corrections for semiempirical QM methods and force fields. J. Chem. Theory Comput. 2010, 6, 3808–3816. [Google Scholar] [CrossRef]
- Kromann, J.C.; Christensen, A.S.; Steinmann, C.; Korth, M.; Jensen, J.H. A third-generation dispersion and third-generation hydrogen bonding corrected PM6 method: PM6-D3H+. Peer J. 2014, 2, 449. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]










| Spherical | Trigonal Planar | Tetrahedral | |||||||
|---|---|---|---|---|---|---|---|---|---|
| F− | Cl− | Br− | I− | NO3− | AcO− | BzO− | HSO4− | ClO4− | |
| I. Radius/Å b | 1.33 | 1.81 | 1.96 | 2.20 | 1.79 | 2.32 | — | 1.90 | 2.00 |
| Napht urea 4a | 2.91 | 2.88 | 2.33 | 1.83 | 2.29 | 3.20 | 3.09 | 2.70 | 1.57 |
| Napht thiourea 4b | 2.73 | 2.00 | 1.23 | 1.11 | 1.40 | 2.79 | 2.61 | 2.05 | 1.18 |
| Pyr urea 4c | 3.31 | 3.19 | 2.78 | 2.23 | 2.67 | 3.42 | 3.45 | 2.97 | 1.99 |
| Solvent | λmax,abs (nm) | ε (M−1 cm−1) | λmax,em (nm) | Stokes Shift a (nm) | τf (ns) | ΦFb | kr (ns−1) | knr (ns−1) | |
|---|---|---|---|---|---|---|---|---|---|
| A | CH2Cl2 | 293 | 372 | 79 | 2.61 | 0.52 | 0.20 | 0.18 | |
| B | CH2Cl2 | 352 | 412 | 60 | 5.70 | 0.14 | 0.025 | 0.15 | |
| 4a | CH2Cl2 | 301 | 375 | 74 | 4.84 | 0.37 | 0.076 | 0.13 | |
| MeCN | 295 | 374 | 79 | 3.67 | 0.35 | 0.095 | 0.18 | ||
| 4c | CH2Cl2 | 283 c | 398 e | 115 | 1.98 g | 0.19 i | ― | ― | |
| 340 d | 498 f | 158 | 25.9 h | ― | ― |
| Spherical | Trigonal Planar | Tetrahedral | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Solvent | F− | Cl− | Br− | NO3− | AcO− | BzO− | HSO4− | ClO4− | ||
| 4a | Abs | CH2Cl2 | 4.34 | 3.74 | 3.43 | 3.51 | 4.23 | 4.28 | 3.65 | 2.69 |
| MeCN | 4.25 | 3.51 | 3.26 | 3.28 | 4.04 | 4.01 | 3.54 | 2.54 | ||
| Emi | CH2Cl2 | 4.20 | 3.78 | 3.29 | 3.44 | 4.07 | 4.13 | 3.45 | 2.65 | |
| MeCN | 4.14 | 3.46 | 3.18 | 3.19 | 3.91 | 4.06 | 3.29 | 2.48 | ||
| 4b | Abs | CH2Cl2 | 3.39 | 3.00 | 2.78 | 2.62 | 3.28 | 3.19 | 2.91 | 2.26 |
| 4c | Abs | CH2Cl2 | 4.46 | 3.97 | 3.65 | 3.60 | 4.29 | 4.37 | 3.80 | 2.92 |
| log Kass | ΔG° (kJ mol−1) | ΔH° (kJ mol−1) | ΔS° (J mol−1K−1) | |
|---|---|---|---|---|
| F− | 4.25/4.14 | −25/−25 | 35/32 | 200/190 |
| AcO− | 4.04/3.91 | −23/−22 | 35/32 | 190/180 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Menezes, F. Anion Binding by Fluorescent Ureido-Hexahomotrioxacalix[3]arene Receptors: An NMR, Absorption and Emission Spectroscopic Study. Molecules 2022, 27, 3247. https://doi.org/10.3390/molecules27103247
Miranda AS, Marcos PM, Ascenso JR, Berberan-Santos MN, Menezes F. Anion Binding by Fluorescent Ureido-Hexahomotrioxacalix[3]arene Receptors: An NMR, Absorption and Emission Spectroscopic Study. Molecules. 2022; 27(10):3247. https://doi.org/10.3390/molecules27103247
Chicago/Turabian StyleMiranda, Alexandre S., Paula M. Marcos, José R. Ascenso, Mário N. Berberan-Santos, and Filipe Menezes. 2022. "Anion Binding by Fluorescent Ureido-Hexahomotrioxacalix[3]arene Receptors: An NMR, Absorption and Emission Spectroscopic Study" Molecules 27, no. 10: 3247. https://doi.org/10.3390/molecules27103247
APA StyleMiranda, A. S., Marcos, P. M., Ascenso, J. R., Berberan-Santos, M. N., & Menezes, F. (2022). Anion Binding by Fluorescent Ureido-Hexahomotrioxacalix[3]arene Receptors: An NMR, Absorption and Emission Spectroscopic Study. Molecules, 27(10), 3247. https://doi.org/10.3390/molecules27103247