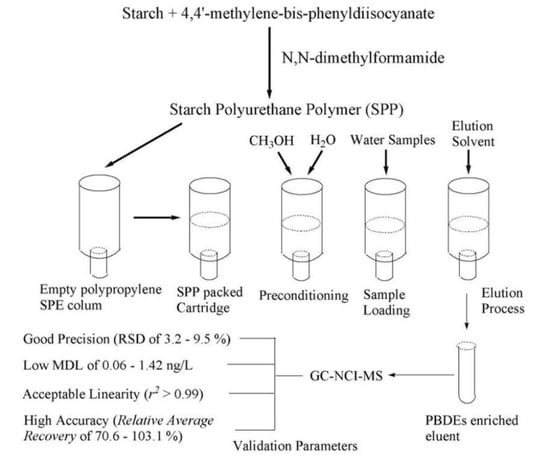
Development of a Solid-Phase Extraction Method Based on Biocompatible Starch Polyurethane Polymers for GC-MS Analysis of Polybrominated Diphenyl Ethers in Ambient Water Samples
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. SPE Procedures
2.3. GC-MS Instrumentation
2.4. Optimization of Relevant SPE Parameters
2.5. Quality Assurance/Quality Control Measures and Water Samples Analysis
3. Results and Discussion
3.1. Adsorbent Design and Adsorbent-Analyte Interaction Mechanism
3.2. Optimization of the SPE Procedure
3.2.1. Effect of the Amount of Adsorbent
3.2.2. Effect of the Elution Solvent and Elution Volume
3.2.3. Effect of Sample Volume
3.2.4. Effect of Methanol Content and Salt Concentration
3.3. Comparison of the Efficiency of the Developed Starch-Based Polymer with Commercially Available SPE Adsorbents
3.4. Validation of the Starch-Based Polymer SPE Method
3.5. Application to Environmental Water
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, Y.-Y.; Tao, F.-M.; Zeng, E.Y. Theoretical Study on the Chemical Properties of Polybrominated Diphenyl Ethers. Chemosphere 2008, 70, 901–907. [Google Scholar] [CrossRef] [PubMed]
- Tseng, L.-H.; Li, M.-H.; Tsai, S.-S.; Lee, C.-W.; Pan, M.-H.; Yao, W.-J.; Hsu, P.-C. Developmental Exposure to Decabromodiphenyl Ether (PBDE 209): Effects on Thyroid Hormone and Hepatic Enzyme Activity in Male Mouse Offspring. Chemosphere 2008, 70, 640–647. [Google Scholar] [CrossRef] [PubMed]
- Dingemans, M.M.L.; van den Berg, M.; Westerink, R.H.S. Neurotoxicity of Brominated Flame Retardants: (In)Direct Effects of Parent and Hydroxylated Polybrominated Diphenyl Ethers on the (Developing) Nervous System. Environ. Health Perspect. 2011, 119, 900–907. [Google Scholar] [CrossRef] [PubMed]
- Besis, A.; Samara, C. Polybrominated Diphenyl Ethers (PBDEs) in the Indoor and Outdoor Environments—A Review on Occurrence and Human Exposure. Environ. Pollut. 2012, 169, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Shi, J.; Sun, J.; Wang, T.; Zeng, L.; Zhu, N.; Jiang, G. Graphene-Assisted Matrix Solid-Phase Dispersion for Extraction of Polybrominated Diphenyl Ethers and Their Methoxylated and Hydroxylated Analogs from Environmental Samples. Anal. Chim. Acta 2011, 708, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Swart, C.; Gantois, F.; Petrov, P.; Entwisle, J.; Goenaga-infante, H.; Nousiainen, M.; Bílsel, M.; Binici, B. Potential Reference Measurement Procedures for PBDE in Surface Water at Levels Required by the EU Water Frame Directive. Talanta 2016, 152, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Smiełowska, M.; Zabiegala, B. Current Trends in Analytical Strategies for the Determination of Polybrominated Diphenyl Ethers (PBDEs) in Samples with Different Matrix Compositions e Part 2: New Approaches to PBDEs Determination. TrAC Trends Anal. Chem. 2020, 132, 115889. [Google Scholar] [CrossRef]
- Smiełowska, M.; Zabiegala, B. Current Trends in Analytical Strategies for Determination of Polybrominated Diphenyl Ethers (PBDEs) in Samples with Different Matrix Compositions e Part 1: Screening of New Developments in Sample Preparation. Trends Anal. Chem. 2020, 132, 115255. [Google Scholar] [CrossRef]
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Guo, Q. QSAR Aided Design and Development of Biopolymer-Based SPE Phase for Liquid Chromatographic Analysis of Polycyclic Aromatic Hydrocarbons in Environmental Water Samples. RSC Adv. 2016, 6, 71596–71611. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, T.; Guan, L. Ultrasound-Assisted Dispersive Liquid–Liquid Microextraction Combined with Gas Chromatography-Mass Spectrometry in Negative Chemical Ionization Mode for the Determination of Polybrominated Diphenyl Ethers in Water. J. Sep. Sci. 2013, 36, 1263–1269. [Google Scholar] [CrossRef]
- Fulara, I.; Czaplicka, M. Methods for Determination of Polybrominated Diphenyl Ethers in Environmental Samples—Review. J. Sep. Sci. 2012, 35, 2075–2087. [Google Scholar] [CrossRef] [PubMed]
- Król, S.; Zabiegała, B.; Namieśnik, J. Determination of Polybrominated Diphenyl Ethers in House Dust Using Standard Addition Method and Gas Chromatography with Electron Capture and Mass Spectrometric Detection. J. Chromatogr. A 2012, 1249, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.P.; Pessah, I.N.; Puschner, B. Simultaneous Determination of Polybrominated Diphenyl Ethers and Polychlorinated Biphenyls by Gas Chromatography-Tandem Mass Spectrometry in Human Serum and Plasma. Talanta 2013, 113, 41–48. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Lee, H.K. Plunger-in-Needle Solid-Phase Microextraction with Graphene-Based Sol-Gel Coating as Sorbent for Determination of Polybrominated Diphenyl Ethers. J. Chromatogr. A 2011, 1218, 4509–4516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Y.; Wu, C.; Xing, J.; Li, J. Polymer-Functionalized Single-Walled Carbon Nanotubes as a Novel Sol-Gel Solid-Phase Micro-Extraction Coated Fiber for Determination of Poly-Brominated Diphenyl Ethers in Water Samples with Gas Chromatography-Electron Capture Detection. Anal. Chem. 2009, 15, 2912–2920. [Google Scholar] [CrossRef] [PubMed]
- Erger, C.; Balsaa, P.; Werres, F.; Schmidt, T.C. Determination of Organic Priority Pollutants in the Low Nanogram-per-Litre Range in Water by Solid-Phase Extraction Disk Combined with Large-Volume Injection/Gas Chromatography–Mass Spectrometry. Anal. Bioanal. Chem. 2013, 405, 5215–5223. [Google Scholar] [CrossRef]
- Marć, M.; Wieczorek, P.P. The Preparation and Evaluation of Core-Shell Magnetic Dummy-Template Molecularly Imprinted Polymers for Preliminary Recognition of the Low-Mass Polybrominated Diphenyl Ethers from Aqueous Solutions. Sci. Total Environ. 2020, 724, 138151. [Google Scholar] [CrossRef]
- Nzangya, J.M.; Ndunda, E.N.; Bosire, G.O.; Martincigh, B.S.; Nyamori, V.O. Polybrominated Diphenyl Ethers (PBDEs) as Emerging Environmental Pollutants: Advances in Sample Preparation and Detection Techniques. In Emerging Contaminants; IntechOpen: London, UK, 2021. [Google Scholar]
- Choodum, A.; Lamthornkit, N.; Boonkanon, C.; Taweekarn, T.; Phatthanawiwat, K.; Sriprom, W.; Limsakul, W.; Chuenchom, L.; Wongniramaikul, W. Greener Monolithic Solid Phase Extraction Biosorbent Based on Calcium Cross-Linked Starch Cryogel Composite Graphene Oxide Nanoparticles for Benzo(a)Pyrene Analysis. Molecules 2021, 26, 6163. [Google Scholar] [CrossRef]
- Wang, Z.; Xiang, B.; Cheng, R.; Li, Y. Behaviors and Mechanism of Acid Dyes Sorption onto Diethylenetriamine-Modified Native and Enzymatic Hydrolysis Starch. J. Hazard. Mater. 2010, 183, 224–232. [Google Scholar] [CrossRef]
- Cheng, R.; Ou, S.; Xiang, B.; Li, Y.; Chang, F.; Li, M. Acyclic Polyamine Modified Starch for Amido Black 10B Removal in Basic Solution. Desalin. Water Treat. 2010, 16, 176–181. [Google Scholar] [CrossRef]
- Ren, Z.; Liu, C.; Zhang, B.; Wu, M.; Tan, Y.; Fang, X.; Yang, P.; Liu, L. Preparation of Amino-Functionalized Starch-Based Adsorbent and Its Adsorption Behavior for Uranyl Ions. J. Radioanal. Nucl. Chem. 2021, 328, 1253–1263. [Google Scholar] [CrossRef]
- Haroon, M.; Wang, L.; Yu, H.; Abbasi, N.M.; Zain-ul-Abdin; Saleem, M.; Khan, R.U.; Ullah, R.S.; Chen, Q.; Wu, J. Chemical Modification of Starch and Its Application as an Adsorbent Material. RSC Adv. 2016, 6, 78264–78285. [Google Scholar] [CrossRef]
- Gupta, A.D.; Rawat, K.P.; Bhadauria, V.; Singh, H. Recent Trends in the Application of Modified Starch in the Adsorption of Heavy Metals from Water: A Review. Carbohydr. Polym. 2021, 269, 117763. [Google Scholar] [CrossRef] [PubMed]
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Diagboya, P.N.; Guo, Q. Mechanism of Dialkyl Phthalates Removal from Aqueous Solution Using γ-Cyclodextrin and Starch Based Polyurethane Polymer Adsorbents. Carbohydr. Polym. 2014, 114, 440–449. [Google Scholar] [CrossRef] [Green Version]
- Okoli, C.P.; Adewuyi, G.O.; Zhang, Q.; Zhu, G.; Wang, C.; Guo, Q. Aqueous Scavenging of Polycyclic Aromatic Hydrocarbons Using Epichlorohydrin, 1,6-Hexamethylene Diisocyanate and 4,4-Methylene Diphenyl Diisocyanate Modified Starch: Pollution Remediation Approach. Arab. J. Chem. 2019, 12, 2760–2773. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Okoli, C.P.; Wang, L.; Liang, T. Adsorption of Nitrophenol Compounds from Aqueous Solution by Cross-Linked Starch-Based Polymers. Desalin. Water Treat. 2014, 55, 1575–1585. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Development of Sustainable Magnetic Polyurethane Polymer Nanocomposite for Abatement of Tetracycline Antibiotics Aqueous Pollution: Response Surface Methodology and Adsorption Dynamics. J. Clean. Prod. 2019, 217, 42–55. [Google Scholar] [CrossRef]
- Okoli, C.P.; Ofomaja, A.E. Degree of Time Dependency of Kinetic Coefficient as a Function of Adsorbate Concentration; New Insights from Adsorption of Tetracycline onto Monodispersed Starch-Stabilized Magnetic Nanocomposite. J. Environ. Manag. 2018, 218, 139–147. [Google Scholar] [CrossRef]
- Zhang, Q.; Liang, T.; Wang, L.; Cai, Y.; Okoli, C.P. Cross-Linked Starch-Based Polymer as an SPE Material for the Determination of Nitrophenols at Trace Levels in Environmental Water. J. Sep. Sci. 2014, 37, 257–264. [Google Scholar] [CrossRef]
- Mafra, G.; García-Valverde, M.; Millán-Santiago, J.; Carasek, E.; Lucena, R.; Cárdenas, S. Returning to Nature for the Design of Sorptive Phases in Solid-Phase Microextraction. Separations 2019, 7, 2. [Google Scholar] [CrossRef] [Green Version]
- Fontana, A.R.; Silva, M.F.; Martínez, L.D.; Wuilloud, R.G.; Altamirano, J.C. Determination of Polybrominated Diphenyl Ethers in Water and Soil Samples by Cloud Point Extraction-Ultrasound-Assisted Back-Extraction-Gas Chromatography–Mass Spectrometry. J. Chromatogr. A 2009, 1216, 4339–4346. [Google Scholar] [CrossRef] [PubMed]
- Byczkiewicz, M.; Jabłoński, M. Determining Polybrominated Diphenyl Ethers in Surface Waters of Western Pomerania Using Gas Chromatography with Electron Capture Detection. Pol. J. Environ. Stud. 2015, 24, 961–968. [Google Scholar] [CrossRef] [Green Version]
- Bacaloni, A.; Callipo, L.; Corradini, E.; Giansanti, P.; Gubbiotti, R.; Samperi, R.; Laganà, A. Liquid Chromatography-Negative Ion Atmospheric Pressure Photoionization Tandem Mass Spectrometry for the Determination of Brominated Flame Retardants in Environmental Water and Industrial Effluents. J. Chromatogr. A 2009, 1216, 6400–6409. [Google Scholar] [CrossRef] [PubMed]








| Congener | SPE Cartridge | Separation Method | Recovery (%) | RSD (%) | Reference |
|---|---|---|---|---|---|
| BDE-47,99,100,153 | LC-18 | HPLC-APPI-MS/MS | 58–92 | 5–22 | [34] |
| BDE-47,99,100,153 | OASIS | HPLC-APPI-MS/MS | 67–93 | 6–14 | [34] |
| BDE-28, 47, 99, 100,153,154,183, 209 | OASIS (This study) | GC-NCI-MS | 69.3–101.5 | 3.2–11.2 | |
| BDE-28, 47, 99, 100,153,154,183, 209 | SPP (This study) | GC-NCI-MS | 71.3–104.2 | 3.6–9.5 | |
| BDE-28, 47, 99, 100, 153, 154 | LLE | GC-µECD | 85–95 | 2.06–3.87 | [33] |
| BDE-47, 99, 100, 153 | CPE-UABE | GC-MS | 96–106 | 4.2–8.5 | [32] |
| Compounds | RSD (%) a n = 5 | LR b (ng L−1) | LOD c (ng L−1) | r2 |
|---|---|---|---|---|
| BDE-28 | 3.6 | 2–100 | 0.06 | 0.9986 |
| BDE-47 | 3.2 | 2–100 | 0.14 | 0.9976 |
| BDE-99 | 6.3 | 2–100 | 0.35 | 0.9942 |
| BDE-100 | 3.9 | 2–100 | 0.58 | 0.9970 |
| BDE-153 | 8.6 | 5–200 | 0.72 | 0.9911 |
| BDE-154 | 6.5 | 5–200 | 1.42 | 0.9944 |
| BDE-183 | 4.7 | 5–200 | 1.23 | 0.9916 |
| BDE-209 | 9.5 | 5–200 | 1.35 | 0.9903 |
| Water Samples | Spiked (ng L−1) | Relative Average Recovery (%) (n = 5) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| BDE-28 | BDE-47 | BDE-99 | BDE-100 | BDE-153 | BDE-154 | BDE-183 | BDE-209 | ||
| River water | 5.0 | 96.0 | 94.4 | 97.2 | 102.6 | 82.2 | 79.2 | 75.2 | 68.2 |
| 50.0 | 102.4 | 95.4 | 104.4 | 92.1 | 84.6 | 78.2 | 76.9 | 67.5 | |
| Lake water | 5.0 | 98.2 | 94.1 | 92.3 | 94.1 | 86.7 | 75.4 | 74.2 | 70.6 |
| 50.0 | 103.1 | 90.2 | 104.1 | 96.5 | 88.5 | 78.5 | 73.2 | 71.2 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Okoli, C.P. Development of a Solid-Phase Extraction Method Based on Biocompatible Starch Polyurethane Polymers for GC-MS Analysis of Polybrominated Diphenyl Ethers in Ambient Water Samples. Molecules 2022, 27, 3253. https://doi.org/10.3390/molecules27103253
Zhang Q, Okoli CP. Development of a Solid-Phase Extraction Method Based on Biocompatible Starch Polyurethane Polymers for GC-MS Analysis of Polybrominated Diphenyl Ethers in Ambient Water Samples. Molecules. 2022; 27(10):3253. https://doi.org/10.3390/molecules27103253
Chicago/Turabian StyleZhang, Qian, and Chukwunonso P. Okoli. 2022. "Development of a Solid-Phase Extraction Method Based on Biocompatible Starch Polyurethane Polymers for GC-MS Analysis of Polybrominated Diphenyl Ethers in Ambient Water Samples" Molecules 27, no. 10: 3253. https://doi.org/10.3390/molecules27103253
APA StyleZhang, Q., & Okoli, C. P. (2022). Development of a Solid-Phase Extraction Method Based on Biocompatible Starch Polyurethane Polymers for GC-MS Analysis of Polybrominated Diphenyl Ethers in Ambient Water Samples. Molecules, 27(10), 3253. https://doi.org/10.3390/molecules27103253