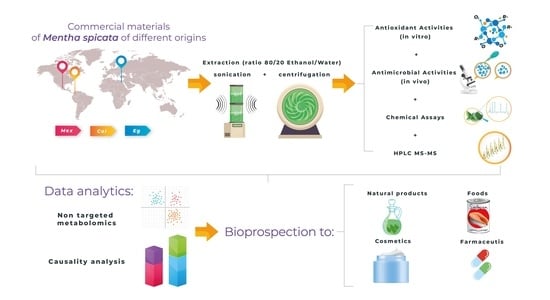
Towards Bioprospection of Commercial Materials of Mentha spicata L. Using a Combined Strategy of Metabolomics and Biological Activity Analyses
Abstract
:1. Introduction
2. Results
2.1. Chemical Measurements of Mentha spicata L.
2.2. Biological Activity Assays
2.2.1. Antioxidant Activities
2.2.2. Antimicrobial Activities
2.3. Metabolomic Analysis
2.4. Causality Analysis
3. Discussion
4. Materials and Methods
4.1. Mentha spicata L. Commercial Materials
4.2. Biological Activities and Chemical Asssays
4.2.1. Preparation of Botanical Extracts of Mentha spicata L.
4.2.2. Determination of Total Phenolic Content (TPC)
4.2.3. DPPH Radical Scavenging Capacity Assay
4.2.4. Reducing Power Assay (FRAP)
4.2.5. ABTS Radical Scavenging Capacity Assay
4.2.6. Quantification of Rosmarinic Acid (RA)
4.3. Antimicrobial Activity in Commercial Materials of Mentha spicata L.
4.4. Metabolic Profiling of Spearmint Based on HPLC-MS and HPLC-MS/MS
Metabolites Annotation
4.5. Causality Analysis between Metabolomic Profiles and Biological Activities
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Akdoğan, M.; Tamer, M.N.; Cüre, E.; Cüre, M.C.; Köroğlu, B.K.; Delibaş, N. Effect of spearmint (Mentha spicata Labiatae) teas on androgen levels in women with hirsutism. Phytother. Res. 2007, 21, 444–447. [Google Scholar] [CrossRef]
- Begaa, S.; Messaoudi, M.; Ouanezar, A.; Hamidatou, L.; Malki, A. Chemical elements of Algerian Mentha spicata L. used in the treatment of digestive system disorders by employing instrumental neutron activation analysis technique. J. Radioanal. Nucl. Chem. 2018, 317, 1107–1112. [Google Scholar] [CrossRef]
- Mahendran, G.; Verma, S.K.; Rahman, L.-U. The traditional uses, phytochemistry and pharmacology of spearmint (Mentha spicata L.): A review. J. Ethnopharmacol. 2021, 278, 114266. [Google Scholar] [CrossRef]
- Cirlini, M.; Mena, P.; Tassotti, M.; Herrlinger, K.A.; Nieman, K.M.; Dall’Asta, C.; Del Rio, D. Phenolic and Volatile Composition of a Dry Spearmint (Mentha spicata L.) Extract. Molecules 2016, 21, 1007. [Google Scholar] [CrossRef] [Green Version]
- Villagran, E.; Henao-Rojas, J.C.; Franco, G. Thermo-Environmental Performance of Four Different Shapes of Solar Greenhouse Dryer with Free Convection Operating Principle and No Load on Product. Fluids 2021, 6, 183. [Google Scholar] [CrossRef]
- Zaidi, S.; Dahiya, P. In vitro antimicrobial activity, phytochemical analysis and total phenolic content of essential oil from Mentha spicata and Mentha piperita. Int. Food Res. J. 2015, 22, 16. [Google Scholar]
- Chauhan, S.S.; Agarwal, R. Evaluation of antibacterial activity of volatile oil from Mentha spicata L. J. Drug Deliv. Ther. 2013, 3, 120–121. [Google Scholar] [CrossRef]
- Wu, Z.; Tan, B.; Liu, Y.; Dunn, J.; Martorell Guerola, P.; Tortajada, M.; Cao, Z.; Ji, P. Chemical Composition and Antioxidant Properties of Essential Oils from Peppermint, Native Spearmint and Scotch Spearmint. Molecules 2019, 24, 2825. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.-L.; Chen, Y.; Li, Z.-J.; Li, X.; Fan, G. Bioactive properties of the aromatic molecules of spearmint (Mentha spicata L.) essential oil: A review. Food Funct. 2022, 13, 3110–3132. [Google Scholar] [CrossRef]
- Eftekhari, A.; Khusro, A.; Ahmadian, E.; Dizaj, S.M.; Hasanzadeh, A.; Cucchiarini, M. Phytochemical and nutra-pharmaceutical attributes of Mentha spp.: A comprehensive review. Arab. J. Chem. 2021, 14, 103106. [Google Scholar] [CrossRef]
- Pearson, W.; Fletcher, R.S.; Kott, L.S.; Hurtig, M.B. Protection against LPS-induced cartilage inflammation and degradation provided by a biological extract of Mentha spicata. BMC Complementary Altern. Med. 2010, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Kee, L.A.; Shori, A.B.; Baba, A.S. Bioactivity and health effects of Mentha spicata. Food Nutr. Metab. 2017, 5, 1–2. [Google Scholar]
- Farnad, N.; Heidari, R.; Aslanipour, B. Phenolic composition and comparison of antioxidant activity of alcoholic extracts of Peppermint (Mentha piperita). J. Food Meas. Charact. 2014, 8, 113–121. [Google Scholar] [CrossRef]
- Elansary, H.O.; Szopa, A.; Kubica, P.; Ekiert, H.; Klimek-Szczykutowicz, M.; El-Ansary, D.O.; Mahmoud, E.A. Polyphenol Profile and Antimicrobial and Cytotoxic Activities of Natural Mentha × piperita and Mentha longifolia Populations in Northern Saudi Arabia. Processes 2020, 8, 479. [Google Scholar] [CrossRef] [Green Version]
- Uribe, E.; Marín, D.; Vega-Gálvez, A.; Quispe-Fuentes, I.; Rodríguez, A. Assessment of vacuum-dried peppermint (Mentha piperita L.) as a source of natural antioxidants. Food Chem. 2016, 190, 559–565. [Google Scholar] [CrossRef]
- Pistelli, L.; Sansone, C.; Smerilli, A.; Festa, M.; Noonan, D.M.; Albini, A.; Brunet, C. MMP-9 and IL-1β as Targets for Diatoxanthin and Related Microalgal Pigments: Potential Chemopreventive and Photoprotective Agents. Mar. Drugs 2021, 19, 354. [Google Scholar] [CrossRef]
- Tominaga, K.; Hongo, N.; Karato, M.; Yamashita, E. Cosmetic benefits of astaxanthin on humans subjects. Acta Biochim. Pol. 2012, 59, 43–47. [Google Scholar] [CrossRef]
- Davinelli, S.; Nielsen, M.E.; Scapagnini, G. Astaxanthin in Skin Health, Repair, and Disease: A Comprehensive Review. Nutrients 2018, 10, 522. [Google Scholar] [CrossRef] [Green Version]
- Mansourabadi, A.H.; Sadeghi, H.M.; Razavi, N.; Rezvani, E. Anti-inflammatory and Analgesic Properties of Salvigenin, Salvia officinalis Flavonoid Extracted. J. Future Nat. Prod. 2016, 2, 31–41. [Google Scholar]
- Alagawany, M.; Abd El-Hack, M.E.; Farag, M.R.; Gopi, M.; Karthik, K.; Malik, Y.S.; Dhama, K. Rosmarinic acid: Modes of action, medicinal values and health benefits. Anim. Health Res. Rev. 2017, 18, 167–176. [Google Scholar] [CrossRef]
- González-Vallinas, M.; Molina, S.; Vicente, G.; de la Cueva, A.; Vargas, T.; Santoyo, S.; García-Risco, M.R.; Fornari, T.; Reglero, G.; Ramírez de Molina, A. Antitumor effect of 5-fluorouracil is enhanced by rosemary extract in both drug sensitive and resistant colon cancer cells. Pharmacol. Res. 2013, 72, 61–68. [Google Scholar] [CrossRef]
- Ngo, Y.L.; Lau, C.H.; Chua, L.S. Review on rosmarinic acid extraction, fractionation and its anti-diabetic potential. Food Chem. Toxicol. 2018, 121, 687–700. [Google Scholar] [CrossRef]
- Caruso, G.; Godos, J.; Privitera, A.; Lanza, G.; Castellano, S.; Chillemi, A.; Bruni, O.; Ferri, R.; Caraci, F.; Grosso, G. Phenolic Acids and Prevention of Cognitive Decline: Polyphenols with a Neuroprotective Role in Cognitive Disorders and Alzheimer’s Disease. Nutrients 2022, 14, 819. [Google Scholar] [CrossRef]
- Liang, C.; Ju, W.; Pei, S.; Tang, Y.; Xiao, Y. Pharmacological Activities and Synthesis of Esculetin and Its Derivatives: A Mini-Review. Molecules 2017, 22, 387. [Google Scholar] [CrossRef] [Green Version]
- Casciaro, B.; Moros, M.; Rivera-Fernández, S.; Bellelli, A.; de la Fuente, J.M.; Mangoni, M.L. Gold-nanoparticles coated with the antimicrobial peptide esculentin-1a(1-21)NH2 as a reliable strategy for antipseudomonal drugs. Acta Biomater. 2017, 47, 170–181. [Google Scholar] [CrossRef] [Green Version]
- Naveed, M.; Hejazi, V.; Abbas, M.; Kamboh, A.A.; Khan, G.J.; Shumzaid, M.; Ahmad, F.; Babazadeh, D.; FangFang, X.; Modarresi-Ghazani, F.; et al. Chlorogenic acid (CGA): A pharmacological review and call for further research. Biomed. Pharmacother. 2018, 97, 67–74. [Google Scholar] [CrossRef]
- Alsaraf, S.; Hadi, Z.; Akhtar, M.J.; Khan, S.A. Chemical profiling, cytotoxic and antioxidant activity of volatile oil isolated from the mint (Mentha spicata L.,) grown in Oman. Biocatal. Agric. Biotechnol. 2021, 34, 102034. [Google Scholar] [CrossRef]
- Yousuf, T.; Akter, R.; Ahmed, J.; Mazumdar, S.; Talukder, D.; Nandi, N.C.; Nurulamin, M. Evaluation of acute oral toxicity, cytotoxicity, antidepressant and antioxidant activities of Japanese mint (Mentha arvensis L.) oil. Phytomedicine Plus 2021, 1, 100140. [Google Scholar] [CrossRef]
- Bardaweel, S.K.; Bakchiche, B.; Alsalamat, H.A.; Rezzoug, M.; Gherib, A.; Flamini, G. Chemical composition, antioxidant, antimicrobial and Antiproliferative activities of essential oil of Mentha spicata L. (Lamiaceae) from Algerian Saharan atlas. BMC Complementary Altern. Med. 2018, 18, 201. [Google Scholar] [CrossRef] [Green Version]
- Golparvar, A.R.; Hadipanah, A.; Mehrabi, A.M. Diversity in chemical composition from two ecotypes of (Mentha longifolia L.) and (Mentha spicata L.) in Iran climatic conditions. J. Biodivers. Environ. Sci. 2015, 6, 26–33. [Google Scholar]
- Devi, A.; Sharma, G. Morphological, phenological and cytological comparison of Mentha longifolia and M. spicata from sub-tropical and temperate regions of Jammu province (J&K). Vegetos 2022, 35, 179–187. [Google Scholar] [CrossRef]
- López-Hernández, F.; Cortés, A.J. Whole Transcriptome Sequencing Unveils the Genomic Determinants of Putative Somaclonal Variation in Mint (Mentha L.). Mol. Sci. 2022, 23, 5291. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Tomé, M.; Jiménez-Monreal, A.M.; García-Jiménez, L.; Almela, L.; García-Diz, L.; Mariscal-Arcas, M.; Murcia, M.A. Assessment of antimicrobial activity of coffee brewed in three different ways from different origins. Eur. Food Res. Technol. 2011, 233, 497. [Google Scholar] [CrossRef]
- Farjana, A.; Zerin, N.; Kabir, M.S. Antimicrobial activity of medicinal plant leaf extracts against pathogenic bacteria. Asian Pac. J. Trop. Dis. 2014, 4, S920–S923. [Google Scholar] [CrossRef]
- Suárez-Quiroz, M.L.; Taillefer, W.; López Méndez, E.M.; González-Ríos, O.; Villeneuve, P.; Figueroa-Espinoza, M.-C. Antibacterial Activity and Antifungal and Anti-Mycotoxigenic Activities Against A spergillus flavus and A. ochraceus of Green Coffee Chlorogenic Acids and Dodecyl Chlorogenates. J. Food Saf. 2013, 33, 360–368. [Google Scholar] [CrossRef]
- Chaves-Ulate, E.; Esquivel-Rodríguez, P. Ácidos clorogénicos presentes en el café: Capacidad antimicrobiana y antioxidante. Agron. Mesoam. 2019, 30, 299–311. [Google Scholar] [CrossRef]
- Serino, E.; Chahardoli, A.; Badolati, N.; Sirignano, C.; Jalilian, F.; Mojarrab, M.; Farhangi, Z.; Rigano, D.; Stornaiuolo, M.; Shokoohinia, Y.; et al. Salvigenin, a Trimethoxylated Flavone from Achillea Wilhelmsii C. Koch, Exerts Combined Lipid-Lowering and Mitochondrial Stimulatory Effects. Antioxidants 2021, 10, 1042. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, Q.; Li, X. Esculetin: A review of its pharmacology and pharmacokinetics. Phytother. Res. 2022, 36, 279–298. [Google Scholar] [CrossRef]
- Wang, X.; Du, J.; Zhou, J. Antibiotic activities of extracts from Prunus mume fruit against food-borne bacterial pathogens and its active components. Ind. Crops Prod. 2019, 133, 409–413. [Google Scholar] [CrossRef]
- Jiménez, N.; Carrillo-Hormaza, L.; Pujol, A.; Álzate, F.; Osorio, E.; Lara-Guzman, O. Antioxidant capacity and phenolic content of commonly used anti-inflammatory medicinal plants in Colombia. Ind. Crops Prod. 2015, 70, 272–279. [Google Scholar] [CrossRef]
- Park, Y.J.; Baek, S.-A.; Choi, Y.; Kim, J.K.; Park, S.U. Metabolic Profiling of Nine Mentha Species and Prediction of Their Antioxidant Properties Using Chemometrics. Molecules 2019, 24, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, R.; Lemos, M.F.; Lemos, M.F.; Martinelli, G.C.; Martins, J.D.L.; da Silva, A.G. Antioxidant and antibacterial activities and composition of Brazilian spearmint (Mentha spicata L.). Ind. Crops Prod. 2013, 50, 408–413. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.), M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Sõukand, R.; Pieroni, A.; Biró, M.; Dénes, A.; Dogan, Y.; Hajdari, A.; Kalle, R.; Reade, B.; Mustafa, B.; Nedelcheva, A.; et al. An ethnobotanical perspective on traditional fermented plant foods and beverages in Eastern Europe. J. Ethnopharmacol. 2015, 170, 284–296. [Google Scholar] [CrossRef]
- Bhatia, H.; Sharma, Y.P.; Manhas, R.K.; Kumar, K. Ethnomedicinal plants used by the villagers of district Udhampur, J&K, India. J. Ethnopharmacol. 2014, 151, 1005–1018. [Google Scholar] [CrossRef]
- Biswas, A.K.; Chatli, M.K.; Sahoo, J. Antioxidant potential of curry (Murraya koenigii L.) and mint (Mentha spicata) leaf extracts and their effect on colour and oxidative stability of raw ground pork meat during refrigeration storage. Food Chem. 2012, 133, 467–472. [Google Scholar] [CrossRef]
- Sierra, K.; Naranjo, L.; Carrillo-Hormaza, L.; Franco, G.; Osorio, E. Spearmint (Mentha spicata L.) Phytochemical Profile: Impact of Pre/Post-Harvest Processing and Extractive Recovery. Molecules 2022, 27, 2243. [Google Scholar] [CrossRef]
- Agudelo, C.; Bravo, K.; Ramírez-Atehortúa, A.; Torres, D.; Carrillo-Hormaza, L.; Osorio, E. Chemical and Skincare Property Characterization of the Main Cocoa Byproducts: Extraction Optimization by RSM Approach for Development of Sustainable Ingredients. Molecules 2021, 26, 7429. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Mesa-Vanegas, A.M.A.; Zapata-Uribe, S.n.; Arana, L.M.; Zapata, I.C.; Monsalve, Z.; Rojano, B.J. Actividad antioxidante de extractos de diferente polaridad de Ageratum conyzoides L. Chem. Lat. Am. Caribb. Bull. Med. Aromat. Plants 2015, 14, 1–10. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsugawa, H.; Cajka, T.; Kind, T.; Ma, Y.; Higgins, B.; Ikeda, K.; Kanazawa, M.; VanderGheynst, J.; Fiehn, O.; Arita, M. MS-DIAL: Data-independent MS/MS deconvolution for comprehensive metabolome analysis. Nat. Methods 2015, 12, 523–526. [Google Scholar] [CrossRef] [PubMed]
- Chong, J.; Xia, J. MetaboAnalystR: An R package for flexible and reproducible analysis of metabolomics data. Bioinformatics 2018, 34, 4313–4314. [Google Scholar] [CrossRef] [Green Version]
- Sofina, E.V. Agricultural land-use optimization by farms based on quality management: Lines of research. Int. J. Qual. Res. 2019, 13, 915. [Google Scholar] [CrossRef]






| No. | RT | m/z | Adduct | Molecular Formula | Anotación | Class |
|---|---|---|---|---|---|---|
| 1 | 5.835 | 119.08387 | [M + H]+ | C9H12 | 7-Methylene-1,3,5-cyclooctatriene | Cycloparaffin |
| 2 | 18.619 | 585.45056 | [M + H]+ | C40H56O3 | Antheraxanthin | Carotenoid |
| 3 | 18.609 | 329.1127 | [M + H]+ | C18H16O6 | Salvigenin | Flavone |
| 4 | 6.296 | 361.091 | [M + H]+ | C18H16O8 | Rosmarinic acid | Caffeic acid ester |
| 5 | 19.208 | 179.10556 | [M + H]+ | C9H6O4 | Esculetin | Hydroxycoumarin |
| 6 | 18.078 | 355.09079 | [M + H]+ | C16H18O9 | Chlorogenic acid | Cinnamate ester |
| Compound | Food/Pharmacy Uses | References |
|---|---|---|
| 7-Methylene-1,3,5-cyclooctatriene | Reports not found. | |
| Antheraxanthin | Skin care. | [16,17,18] |
| Salvigenin | Antiinflammatory and analgesic properties. | [19] |
| Rosmarinic acid | Antibacterial, antioxidant, anticancer, antiinflammatory, immunomodulatory, and coadjuvant activities in the treatment of cancer, diabetes, neuroprotective, and prevention of cognitive decline in Alzheimer’s disease. | [20,21,22,23] |
| Esculetin | Anti-inflamatory, anticoagulant, liver-protection, antidiabetic, antioxidant, antitumor, and UV-filters. | [24,25] |
| Chlorogenic acid | High potential antioxidant, hepatoprotective, cardioprotective, anti-inflammatory, antipyretic, neuroprotective, anti-obesity, antiviral, antimicrobial, anti-hypertension, and free radicals. | [26] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Henao-Rojas, J.C.; Osorio, E.; Isaza, S.; Madronero-Solarte, I.A.; Sierra, K.; Zapata-Vahos, I.C.; Betancur-Pérez, J.F.; Arboleda-Valencia, J.W.; Gallego, A.M. Towards Bioprospection of Commercial Materials of Mentha spicata L. Using a Combined Strategy of Metabolomics and Biological Activity Analyses. Molecules 2022, 27, 3559. https://doi.org/10.3390/molecules27113559
Henao-Rojas JC, Osorio E, Isaza S, Madronero-Solarte IA, Sierra K, Zapata-Vahos IC, Betancur-Pérez JF, Arboleda-Valencia JW, Gallego AM. Towards Bioprospection of Commercial Materials of Mentha spicata L. Using a Combined Strategy of Metabolomics and Biological Activity Analyses. Molecules. 2022; 27(11):3559. https://doi.org/10.3390/molecules27113559
Chicago/Turabian StyleHenao-Rojas, Juan Camilo, Edison Osorio, Stephanie Isaza, Inés Amelia Madronero-Solarte, Karina Sierra, Isabel Cristina Zapata-Vahos, Jhon Fredy Betancur-Pérez, Jorge W. Arboleda-Valencia, and Adriana M. Gallego. 2022. "Towards Bioprospection of Commercial Materials of Mentha spicata L. Using a Combined Strategy of Metabolomics and Biological Activity Analyses" Molecules 27, no. 11: 3559. https://doi.org/10.3390/molecules27113559
APA StyleHenao-Rojas, J. C., Osorio, E., Isaza, S., Madronero-Solarte, I. A., Sierra, K., Zapata-Vahos, I. C., Betancur-Pérez, J. F., Arboleda-Valencia, J. W., & Gallego, A. M. (2022). Towards Bioprospection of Commercial Materials of Mentha spicata L. Using a Combined Strategy of Metabolomics and Biological Activity Analyses. Molecules, 27(11), 3559. https://doi.org/10.3390/molecules27113559