Exploring the Interfacial Reaction of Nano Al/CuO Energetic Films through Thermal Analysis and Ab Initio Molecular Dynamics Simulation
Abstract
:1. Introduction
2. Results and Discussion
2.1. Thermal Analysis of Nano Al/CuO Energetic Composite Films
2.2. Time Evolution of Atomic Structures
2.3. Reaction Front Propagation
2.4. Change in the Helmholtz Free Energy during Preparation
3. Experimental Section
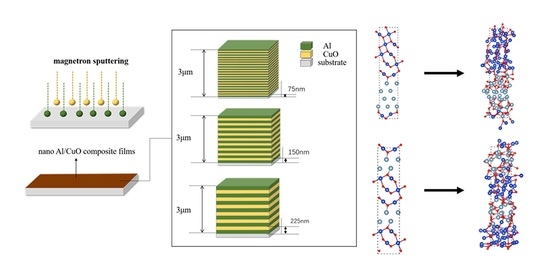
3.1. Preparation of Nano Al/CuO Energetic Composite Films
3.2. Thermal Property Analysis
3.3. Computational Methodology
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Adams, D.P. Reactive multilayers fabricated by vapor deposition: A critical review. Thin Solid Film. 2015, 576, 98–128. [Google Scholar] [CrossRef] [Green Version]
- Rossi, C.; Zhang, K.; Esteve, D.; Alphonse, P.; Tailhades, P.; Vahlas, C. Nanoenergetic Materials for MEMS: A Review. J. Microelectromechanical Syst. 2007, 16, 919–931. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Torabi, M.; Lu, J.; Shen, R.Q.; Zhang, K.L. Nanostructured Energetic Composites: Synthesis, Ignition/Combustion Modeling, and Applications. ACS Appl. Mater. Interfaces 2014, 6, 3058–3074. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Liu, P.J.; He, G.Q.; Gozin, M.; Yan, Q.L. Highly Reactive Metastable Intermixed Composites (MICs): Preparation and Characterization. Adv. Mater. 2018, 30, e1706293. [Google Scholar] [CrossRef] [PubMed]
- Salvagnac, L.; Assie-Souleille, S.; Rossi, C. Layered Al/CuO Thin Films for Tunable Ignition and Actuations. Nanomaterials 2020, 10, 2009. [Google Scholar] [CrossRef]
- Rossi, C. Two Decades of Research on Nano-Energetic Materials. Propellants Explos. Pyrotech. 2014, 39, 323–327. [Google Scholar] [CrossRef]
- Tai, Y.; Xu, J.B.; Wang, F.; Dai, J.; Zhang, W.; Ye, Y.H.; Shen, R.Q. Experimental and modeling investigation on the self-propagating combustion behavior of Al-MoO3 reactive multilayer films. J. Appl. Phys. 2018, 123, 235302. [Google Scholar] [CrossRef]
- Dutro, G.M.; Yetter, R.A.; Risha, G.A.; Son, S.F. The effect of stoichiometry on the combustion behavior of a nanoscale Al/MoO(3) thermite. Proc. Combust. Inst. 2009, 32, 1921–1928. [Google Scholar] [CrossRef]
- Zhou, X.; Zhu, Y.; Ke, X.; Zhang, K.L. Exploring the solid-state interfacial reaction of Al/Fe2O3 nanothermites by thermal analysis. J. Mater. Sci. 2019, 54, 4115–4123. [Google Scholar] [CrossRef]
- Zhang, W.C.; Yin, B.Q.; Shen, R.Q.; Ye, J.H.; Thomas, J.A.; Chao, Y.M. Significantly Enhanced Energy Output from 3D Ordered Macroporous Structured Fe2O3/Al Nanothermite Film. ACS Appl. Mater. Interfaces 2013, 5, 239–242. [Google Scholar] [CrossRef]
- Firat, Y.E. Influence of current density on Al:NiO thin films via electrochemical deposition: Semiconducting and electrochromic properties. Mat. Sci. Semicon. Proc. 2020, 109, 104958. [Google Scholar] [CrossRef]
- Yan, Y.C.; Shi, W.; Jiang, H.C.; Xiong, J.; Zhang, W.L.; Li, Y.R. Fabrication and Characterization of Al/NiO Energetic Nanomultilayers. J. Nanomater. 2015, 2015, 4. [Google Scholar] [CrossRef]
- Baras, F.; Turlo, V.; Politano, O.; Vadchenko, S.G.; Rogachev, A.S.; Mukasyan, A.S. SHS in Ni/Al Nanofoils: A Review of Experiments and Molecular Dynamics Simulations. Adv. Eng. Mater. 2018, 20, 1800091. [Google Scholar] [CrossRef]
- Dreizin, E.L. Metal-based reactive nanomaterials. Prog. Energy Combust. Sci. 2009, 35, 141–167. [Google Scholar] [CrossRef]
- Knepper, R.; Snyder, M.R.; Fritz, G.; Fisher, K.; Knio, O.M.; Weihs, T.P. Effect of varying bilayer spacing distribution on reaction heat and velocity in reactive Al/Ni multilayers. J. Appl. Phys. 2009, 105, 083504. [Google Scholar] [CrossRef]
- Fu, K.K.; Sheppard, L.; Chang, L.; An, X.H.; Yang, C.H.; Ye, L. Comparative study on plasticity and fracture behaviour of Ti/Al multilayers. Tribol. Int. 2018, 126, 344–351. [Google Scholar] [CrossRef]
- Sen, S.; Lake, M.; Wilden, J.; Schaaf, P. Synthesis and characterization of Ti/Al reactive multilayer films with various molar ratios. Thin Solid Film. 2017, 631, 99–105. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Wang, Y.; Ai, M.T.; Jiang, H.C.; Yan, Y.C.; Zhao, X.H.; Wang, L.; Zhang, W.L.; Li, Y.R. Reactive B/Ti Nano-Multilayers with Superior Performance in Plasma Generation. ACS Appl. Mater. Interfaces 2018, 10, 21582–21589. [Google Scholar] [CrossRef]
- Reeves, R.V.; Rodriguez, M.A.; Jones, E.D.; Adams, D.P. Condensed-Phase and Oxidation Reaction Behavior of Ti/2B Foils in Varied Gaseous Environments. J. Phys. Chem. C 2012, 116, 17904–17912. [Google Scholar] [CrossRef]
- Blobaum, K.J.; Reiss, M.E.; Plitzko, J.M.; Weihs, T.P. Deposition and characterization of a self-propagating CuOx/Al thermite reaction in a multilayer foil geometry. J. Appl. Phys. 2003, 94, 2915–2922. [Google Scholar] [CrossRef]
- Manesh, N.A.; Basu, S.; Kumar, R. Experimental flame speed in multi-layered nano-energetic materials. Combust. Flame 2010, 157, 476–480. [Google Scholar] [CrossRef]
- Kwon, J.; Ducere, J.M.; Alphonse, P.; Bahrami, M.; Petrantoni, M.; Veyan, J.F.; Tenailleau, C.; Esteve, A.; Rossi, C.; Chabal, Y.J. Interfacial chemistry in Al/CuO reactive nanomaterial and its role in exothermic reaction. ACS Appl. Mater. Interfaces 2013, 5, 605–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Tai, Y.; Ru, C.; Dai, J.; Ye, Y.; Shen, R.; Zhu, P. Tuning the Ignition Performance of a Microchip Initiator by Integrating Various Al/MoO3 Reactive Multilayer Films on a Semiconductor Bridge. ACS Appl Mater Interfaces 2017, 9, 5580–5589. [Google Scholar] [CrossRef] [PubMed]
- Azadmanjiri, J.; Berndt, C.C.; Wang, J.; Kapoora, A.; Srivastava, V.K. Nanolaminated composite materials: Structure, interface role and applications. RSC Adv. 2016, 6, 109361–109385. [Google Scholar] [CrossRef]
- Taton, G.; Lagrange, D.; Conedera, V.; Renaud, L.; Rossi, C. Micro-chip initiator realized by integrating Al/CuO multilayer nanothermite on polymeric membrane. J. Micromechanics Microengineering 2013, 23, 105009. [Google Scholar] [CrossRef]
- Zhu, P.; Shen, R.; Ye, Y.; Fu, S.; Li, D. Characterization of Al/CuO nanoenergetic multilayer films integrated with semiconductor bridge for initiator applications. J. Appl. Phys. 2013, 113, 184505. [Google Scholar] [CrossRef]
- Ahn, J.Y.; Kim, S.B.; Kim, J.H.; Jang, N.S.; Kim, D.H.; Lee, H.W.; Kim, J.M.; Kim, S.H. A micro-chip initiator with controlled combustion reactivity realized by integrating Al/CuO nanothermite composites on a microhotplate platform. J. Micromechanics Microengineering 2016, 26, 015002. [Google Scholar] [CrossRef]
- Yan, Y.; Shi, W.; Jiang, H.; Xiong, J.; Zhang, W.; Li, Y. Characteristics of the Energetic Igniters Through Integrating Al/NiO Nanolaminates on Cr Film Bridge. Nanoscale Res. Lett. 2015, 10, 504. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Shi, W.; Jiang, H.; Cai, X.; Deng, X.; Xiong, J.; Zhang, W. Characteristics of the Energetic Igniters Through Integrating B/Ti Nano-Multilayers on TaN Film Bridge. Nanoscale Res. Lett. 2015, 10, 934. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Shen, R.Q.; Ye, Y.H.; Zhu, P.; Hu, Y.; Wu, L.Z. Influence of Al/CuO reactive multilayer films additives on exploding foil initiator. J. Appl. Phys. 2011, 110, 094505. [Google Scholar] [CrossRef]
- Glavier, L.; Taton, G.; Ducéré, J.-M.; Baijot, V.; Pinon, S.; Calais, T.; Estève, A.; Djafari Rouhani, M.; Rossi, C. Nanoenergetics as pressure generator for nontoxic impact primers: Comparison of Al/Bi2O3, Al/CuO, Al/MoO3 nanothermites and Al/PTFE. Combust. Flame 2015, 162, 1813–1820. [Google Scholar] [CrossRef]
- Fu, S.; Shen, R.; Zhu, P.; Ye, Y. Metal–interlayer–metal structured initiator containing Al/CuO reactive multilayer films that exhibits improved ignition properties. Sens. Actuators A Phys. 2019, 292, 198–204. [Google Scholar] [CrossRef]
- Shi, A.; Zhang, W.; Shen, R. Self-propagating combustion simulation of sputter-deposited nano-energetic multilayer films. J. Phys. Conf. Ser. 2021, 1721, 012003. [Google Scholar] [CrossRef]
- Wang, H.; Julien, B.; Kline, D.J.; Alibay, Z.; Rehwoldt, M.C.; Rossi, C.; Zachariah, M.R. Probing the Reaction Zone of Nanolaminates at ∼μs Time and ∼μm Spatial Resolution. J. Phys. Chem. C 2020, 124, 13679–13687. [Google Scholar] [CrossRef]
- Esteve, A.; Lahiner, G.; Julien, B.; Vivies, S.; Richard, N.; Rossi, C. How Thermal Aging Affects Ignition and Combustion Properties of Reactive Al/CuO Nanolaminates: A Joint Theoretical/Experimental Study. Nanomaterials 2020, 10, 2087. [Google Scholar] [CrossRef]
- Brotman, S.; Rouhani, M.D.; Rossi, C.; Estève, A. A condensed phase model of the initial Al/CuO reaction stage to interpret experimental findings. J. Appl. Phys. 2019, 125, 035102. [Google Scholar] [CrossRef]
- Wang, H.; Biswas, P.; Zachariah, M.R. Direct Imaging and Simulation of the Interface Reaction of Metal/Metal Oxide Nanoparticle Laminates. J. Phys. Chem. C 2022, 126, 8684–8691. [Google Scholar] [CrossRef]
- Rossi, C. Engineering of Al/CuO Reactive Multilayer Thin Films for Tunable Initiation and Actuation. Propell. Explos. Pyrot. 2019, 44, 94–108. [Google Scholar] [CrossRef] [Green Version]
- Xiong, G.; Yang, C.; Feng, S.; Zhu, W. Ab initio molecular dynamics studies on the transport mechanisms of oxygen atoms in the adiabatic reaction of Al/CuO nanothermite. Chem. Phys. Lett. 2020, 745, 137278. [Google Scholar] [CrossRef]
- Tichtchenko, E.; Esteve, A.; Rossi, C. Modeling the self-propagation reaction in heterogeneous and dense media: Application to Al/CuO thermite. Combust. Flame 2021, 228, 173–183. [Google Scholar] [CrossRef]
- Nicollet, A.; Lahiner, G.; Belisario, A.; Souleille, S.; Djafari-Rouhani, M.; Estève, A.; Rossi, C. Investigation of Al/CuO multilayered thermite ignition. J. Appl. Phys. 2017, 121, 034503. [Google Scholar] [CrossRef] [Green Version]
- Julien, B.; Cure, J.; Salvagnac, L.; Josse, C.; Esteve, A.; Rossi, C. Integration of Gold Nanoparticles to Modulate the Ignitability of Nanothermite Films. ACS Appl. Nano Mater. 2020, 3, 2562–2572. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, J.; Wang, C.; Yang, T.; Ye, Y.; Shen, R. Ignition characteristics of energetic nichrome bridge initiator based on Al/CuO reactive multilayer films under capacitor discharge and constant current conditions. Sens. Actuators A Phys. 2020, 313, 112200. [Google Scholar] [CrossRef]
- Wu, Q.; Xiong, G.; Zhu, W.; Xiao, H. How does low temperature coupled with different pressures affect initiation mechanisms and subsequent decompositions in nitramine explosive HMX? Phys. Chem. Chem. Phys. 2015, 17, 22823–22831. [Google Scholar] [CrossRef]
- Abdallah, I.; Zapata, J.; Lahiner, G.; Warot-Fonrose, B.; Cure, J.; Chabal, Y.; Esteve, A.; Rossi, C. Structure and Chemical Characterization at the Atomic Level of Reactions in Al/CuO Multilayers. ACS Appl. Energy Mater. 2018, 1, 1762–1770. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef] [Green Version]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef] [Green Version]
- Kresse, G.; Hafner, J. Ab initio molecular dynamics for open-shell transition metals. Phys. Rev. B Condens. Matter. 1993, 48, 13115–13118. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient Iterative Schemes for Ab Initio Total-Energy Calculations Using a Plane-Wave Basis Set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865, Erratum in Phys. Rev. Lett. 1997, 78, 1396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blochl, P.E. Projector augmented-wave method. Phys. Rev. B Condens. Matter. 1994, 50, 17953–17979. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Anisimov, V.I.; Zaanen, J.; Andersen, O.K. Condensed matter. Band Theory and Mott Insulators: Hubbard U Instead of Stoner I. Phys. Rev. B 1991, 44, 943–954. [Google Scholar] [CrossRef] [Green Version]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188. [Google Scholar] [CrossRef]
- Nosé, S. A Molecular Dynamics Method for Simulations in the Canonical Ensemble. Mol. Phys. 1984, 52, 255–268. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]







| Sample Number | Modulation Period /nm | Number of Interface Layers | DSC Heat Quantity /(J·g−1) | DSC Heat Release/Theoretical Heat Release |
|---|---|---|---|---|
| SAMPLE1 | 75 | 79 | 1660 | 47% |
| SAMPLE2 | 150 | 39 | 1845 | 52% |
| SAMPLE3 | 225 | 25 | 1956 | 55% |
| Upper Interface | Lower Interface | |||
|---|---|---|---|---|
| Velocity (m/s) | Time of Duration (ps) | Velocity (m/s) | Time of Duration (ps) | |
| One-modulation-period structure | 67.5 | 0.4320 | 440.0 | 0.1400 |
| Two-modulation-period structure | 182.1 | 0.0670 | 681.7 | 0.0875 |
| Sample Number | Modulation Period /nm | Single Al Thickness /nm | Single CuO Thickness /nm | Number of Modulation Period | Total Thickness /nm |
|---|---|---|---|---|---|
| SAMPLE1 | 75 | 25 | 50 | 40 | 3000 |
| SAMPLE2 | 150 | 50 | 100 | 20 | 3000 |
| SAMPLE3 | 225 | 75 | 150 | 13 | 3000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, A.; Zheng, H.; Chen, Z.; Zhang, W.; Zhou, X.; Rossi, C.; Shen, R.; Ye, Y. Exploring the Interfacial Reaction of Nano Al/CuO Energetic Films through Thermal Analysis and Ab Initio Molecular Dynamics Simulation. Molecules 2022, 27, 3586. https://doi.org/10.3390/molecules27113586
Shi A, Zheng H, Chen Z, Zhang W, Zhou X, Rossi C, Shen R, Ye Y. Exploring the Interfacial Reaction of Nano Al/CuO Energetic Films through Thermal Analysis and Ab Initio Molecular Dynamics Simulation. Molecules. 2022; 27(11):3586. https://doi.org/10.3390/molecules27113586
Chicago/Turabian StyleShi, Anran, Han Zheng, Zhiyi Chen, Wei Zhang, Xiang Zhou, Carole Rossi, Ruiqi Shen, and Yinghua Ye. 2022. "Exploring the Interfacial Reaction of Nano Al/CuO Energetic Films through Thermal Analysis and Ab Initio Molecular Dynamics Simulation" Molecules 27, no. 11: 3586. https://doi.org/10.3390/molecules27113586
APA StyleShi, A., Zheng, H., Chen, Z., Zhang, W., Zhou, X., Rossi, C., Shen, R., & Ye, Y. (2022). Exploring the Interfacial Reaction of Nano Al/CuO Energetic Films through Thermal Analysis and Ab Initio Molecular Dynamics Simulation. Molecules, 27(11), 3586. https://doi.org/10.3390/molecules27113586