Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line
Abstract
:1. Introduction
2. Methods
3. Triple-Negative Breast Cancer
3.1. Classification of Triple-Negative Breast
3.2. Targeted Therapy of Triple-Negative Breast Cancer
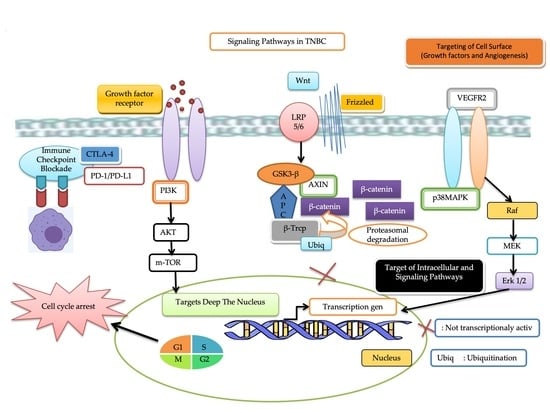
3.2.1. Immune Checkpoint Blockade
Programmed Cell Death Protein 1 (PD-1) and Programmed Death-Ligand 1 (PDL-1)
Cytotoxic T Lymphocyte-Associated Protein 4 (CTLA-4)
Lymphocyte Activation Gene 3 (LAG-3)
T Cell Immunoglobulin and Mucin-Domain Containing-3 (TIM-3)
Hedgehog (Hh) Signaling Pathway
3.2.2. Target Deep the Nucleus
Breast Cancer Susceptibility Gene (BRCA) and Platinum-Based Treatment
Poly-ADP Ribose-Polymerases (PARP)
Histone Deacetylase (HDAC)
p53
3.2.3. Targeting of Intracellular and Signaling Pathways
Androgen Receptor
Heat Shock Protein 90 (HSP90)
Cyclin-Dependent Kinases (CDKs)
Phosphoinositide 3-Kinase (PI3K)/AKT/Mammalian Target of Rapamycin (mTOR) Pathway
RAF-MEK-ERK Pathway
Janus Kinase (JAK)
Signal Transducer and Activator of Transcription 3 (STAT-3)
Wnt/β-Catenin Signaling Pathway
3.2.4. Targeting of Cell Surface
Vascular Endothelial Growth Factor Receptor 2 (VEGFR2)
Epidermal Growth Factor Receptor (EGFR)
Fibroblast Growth Factor Receptor (FGFR)
Trophoblast Antigen 2 (Trop-2) Inhibitor
Glycoprotein Non-Metastatic B (GPNMB)
4. Natural Compounds for TNBC Treatments
4.1. Luteolin
4.2. α-Mangostin
4.3. Piperine
4.4. Silibinin
4.5. Apigenin
4.6. Quercetin
4.7. Fisetin
4.8. Resveratrol
4.9. Genistein
4.10. (10)-Gingerol
4.11. Chalcones
4.12. Berberine
4.13. Curcumin
4.14. Epigallocatechin Gallate
4.15. Cyanidin-3-o-Glucoside
4.16. Glycyrrhizin
4.17. Ilamycin E
4.18. Schisandrin A
4.19. Ampelopsin E
4.20. Aurantoside C
4.21. Amyris texana
4.22. Sequesterpenoid (Tussilago farfara)
4.23. Diterpen Jatrophone
4.24. Naringin/Flavonoid
4.25. Myrothamnus flabelli folius
4.26. Cryptotanshinone
4.27. Curcuma longa
4.28. Ganoderma lucidum
4.29. Astragalus membranaceus
4.30. Vanicoside B
4.31. Eupalinolide J
4.32. Chantaridin
4.33. Cucurbitacin E
5. Future and Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Anastasiadi, Z.; Lianos, G.D.; Ignatiadou, E.; Harissis, H.V.; Mitsis, M. Breast cancer in young women: An overview. Updates Surg. 2017, 69, 313–317. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhan, Z.; Yin, X.; Fu, S.; Deng, X. Targeted Therapeutic Strategies for Triple-Negative Breast Cancer. Front. Oncol. 2021, 11, 4517. [Google Scholar] [CrossRef]
- Metzger-Filho, O.; Tutt, A.; de Azambuja, E.; Saini, K.S.; Viale, G.; Loi, S.; Bradbury, I.; Bliss, J.M.; Azim, H.A., Jr.; Ellis, P. Dissecting the heterogeneity of triple-negative breast cancer. J. Clin. Oncol. 2012, 30, 1879–1887. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Siddharth, S.; Sharma, D. Triple Negative Breast Cancer: A Mountain Yet to Be Scaled Despite the Triumphs. Cancers 2021, 13, 3697. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Gao, Y.; Zhang, J.; Wang, L.; Wang, B.; Cao, J.; Shao, Z.; Wang, Z. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer 2018, 18, 446. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Risk factors for distant metastasis of patients with primary triple-negative breast cancer. Biosci. Rep. 2019, 39, BSR20190288. [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef] [PubMed]
- Marino, N.; Woditschka, S.; Reed, L.T.; Nakayama, J.; Mayer, M.; Wetzel, M.; Steeg, P.S. Breast cancer metastasis: Issues for the personalization of its prevention and treatment. Am. J. Pathol. 2013, 183, 1084–1095. [Google Scholar] [CrossRef] [Green Version]
- Gupta, G.K.; Collier, A.L.; Lee, D.; Hoefer, R.A.; Zheleva, V.; Siewertsz van Reesema, L.L.; Tang-Tan, A.M.; Guye, M.L.; Chang, D.Z.; Winston, J.S.; et al. Perspectives on Triple-Negative Breast Cancer: Current Treatment Strategies, Unmet Needs, and Potential Targets for Future Therapies. Cancers 2020, 12, 2392. [Google Scholar] [CrossRef]
- El Sayed, R.; El Jamal, L.; El Iskandarani, S.; Kort, J.; Abdel Salam, M.; Assi, H. Endocrine and targeted therapy for hormone-receptor-positive, HER2-negative advanced breast cancer: Insights to sequencing treatment and overcoming resistance based on clinical trials. Front. Oncol. 2019, 9, 510. [Google Scholar] [CrossRef] [PubMed]
- Al-Mahmood, S.; Sapiezynski, J.; Garbuzenko, O.B.; Minko, T. Metastatic and triple-negative breast cancer: Challenges and treatment options. Drug Deliv. Transl. Res. 2018, 8, 1483–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gámez-Pozo, A.; Trilla-Fuertes, L.; Prado-Vázquez, G.; Chiva, C.; López-Vacas, R.; Nanni, P.; Berges-Soria, J.; Grossmann, J.; Díaz-Almirón, M.; Ciruelos, E.; et al. Prediction of adjuvant chemotherapy response in triple negative breast cancer with discovery and targeted proteomics. PLoS ONE 2017, 12, e0178296. [Google Scholar] [CrossRef]
- Yao, Y.; Chu, Y.; Xu, B.; Hu, Q.; Song, Q. Radiotherapy after surgery has significant survival benefits for patients with triple-negative breast cancer. Cancer Med. 2019, 8, 554–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahn, S.G.; Kim, S.J.; Kim, C.; Jeong, J. Molecular classification of triple-negative breast cancer. J. Breast Cancer 2016, 19, 223–230. [Google Scholar] [CrossRef]
- Hubalek, M.; Czech, T.; Müller, H. Biological Subtypes of Triple-Negative Breast Cancer. Breast Care 2017, 12, 8–14. [Google Scholar] [CrossRef] [Green Version]
- Hirshfield, K.M.; Ganesan, S. Triple-negative breast cancer: Molecular subtypes and targeted therapy. Curr. Opin. Obstet. Gynecol. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Ashraf, M.A. Phytochemicals as potential anticancer drugs: Time to ponder nature’s bounty. BioMed Res. Int. 2020, 2020, 8602879. [Google Scholar] [CrossRef]
- Saldanha, S.N.; Tollefsbol, T.O. The role of nutraceuticals in chemoprevention and chemotherapy and their clinical outcomes. J. Oncol. 2012, 2012, 192464. [Google Scholar] [CrossRef] [Green Version]
- De, P.; Carlson, J.H.; Wu, H.; Marcus, A.; Leyland-Jones, B.; Dey, N. Wnt-beta-catenin pathway signals metastasis-associated tumor cell phenotypes in triple negative breast cancers. Oncotarget 2016, 7, 43124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Bhaijee, F.; Ishaq, N.; Pepper, D.J.; Backus, K.; Brown, A.S.; Zhou, X.; Miele, L. Correlation of Notch1, pAKT and nuclear NF-κB expression in triple negative breast cancer. Am. J. Cancer Res. 2013, 3, 230. [Google Scholar]
- Costa, R.L.; Han, H.S.; Gradishar, W.J. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: A review. Breast Cancer Res. Treat. 2018, 169, 397–406. [Google Scholar] [CrossRef]
- Santoni, M.; Romagnoli, E.; Saladino, T.; Foghini, L.; Guarino, S.; Capponi, M.; Giannini, M.; Cognigni, P.D.; Ferrara, G.; Battelli, N. Triple negative breast cancer: Key role of tumor-associated macrophages in regulating the activity of anti-PD-1/PD-L1 agents. Biochim. Biophys. Acta-Rev. Cancer 2018, 1869, 78–84. [Google Scholar] [CrossRef]
- Stovgaard, E.S.; Kümler, I.; List-Jensen, K.; Roslind, A.; Christensen, I.J.; Høgdall, E.; Nielsen, D.; Balslev, E. Prognostic and Clinicopathologic Associations of LAG-3 Expression in Triple-negative Breast Cancer. Appl. Immunohistochem. Mol. Morphol. 2021, 30, 62–71. [Google Scholar] [CrossRef]
- Peng, Z.; Su, P.; Yang, Y.; Yao, X.; Zhang, Y.; Jin, F.; Yang, B. Identification of CTLA-4 associated with tumor microenvironment and competing interactions in triple negative breast cancer by co-expression network analysis. J. Cancer 2020, 11, 6365. [Google Scholar] [CrossRef] [PubMed]
- McDaniel, J.M.; Varley, K.E.; Gertz, J.; Savic, D.S.; Roberts, B.S.; Bailey, S.K.; Shevde, L.A.; Ramaker, R.C.; Lasseigne, B.N.; Kirby, M.K. Genomic regulation of invasion by STAT3 in triple negative breast cancer. Oncotarget 2017, 8, 8226. [Google Scholar] [CrossRef] [Green Version]
- El Guerrab, A.; Bamdad, M.; Kwiatkowski, F.; Bignon, Y.-J.; Penault-Llorca, F.; Aubel, C. Anti-EGFR monoclonal antibodies and EGFR tyrosine kinase inhibitors as combination therapy for triple-negative breast cancer. Oncotarget 2016, 7, 73618. [Google Scholar] [CrossRef] [Green Version]
- Khoury, K.; Feldman, R.; Pohlmann, P.R.; Heeke, A.L.; Gatalica, Z.; Veloso, Y.; Vidal, G.A.; Schwartzberg, L.S.; Swain, S.M.; Isaacs, C. Molecular characterization of trophoblast cell surface antigen 2 (Trop-2) positive triple negative breast cancer (TNBC). J. Clin. Oncol. 2019, 37, e14651. [Google Scholar] [CrossRef]
- Nagaria, T.S.; Shi, C.; Leduc, C.; Hoskin, V.; Sikdar, S.; Sangrar, W.; Greer, P.A. Combined targeting of Raf and Mek synergistically inhibits tumorigenesis in triple negative breast cancer model systems. Oncotarget 2017, 8, 80804. [Google Scholar] [CrossRef] [Green Version]
- Song, X.; Liu, Z.; Yu, Z. EGFR promotes the development of triple negative breast cancer through JAK/STAT3 Signaling. Cancer Manag. Res. 2020, 12, 703. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-H.; Chu, P.-Y.; Chen, J.-L.; Huang, C.-T.; Huang, C.-C.; Tsai, Y.F.; Wang, Y.-L.; Lien, P.-J.; Tseng, L.-M.; Liu, C.-Y. Expression pattern and prognostic impact of glycoprotein non-metastatic B (GPNMB) in triple-negative breast cancer. Sci. Rep. 2021, 11, 12171. [Google Scholar] [CrossRef] [PubMed]
- Habib, J.G.; O’Shaughnessy, J.A. The hedgehog pathway in triple-negative breast cancer. Cancer Med. 2016, 5, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Varghese, E.; Samuel, S.M.; Abotaleb, M.; Cheema, S.; Mamtani, R.; Büsselberg, D. The “Yin and Yang” of natural compounds in anticancer therapy of triple-negative breast cancers. Cancers 2018, 10, 346. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Zhang, Q.; Yu, L.; Zhu, J.; Cao, Y.; Gao, X. The signaling pathways and targets of traditional Chinese medicine and natural medicine in triple-negative breast cancer. J. Ethnopharmacol. 2020, 264, 113249. [Google Scholar] [CrossRef]
- Thakuri, P.S.; Gupta, M.; Singh, S.; Joshi, R.; Glasgow, E.; Lekan, A.; Agarwal, S.; Luker, G.D.; Tavana, H. Phytochemicals inhibit migration of triple negative breast cancer cells by targeting kinase signaling. BMC Cancer 2020, 20, 4. [Google Scholar] [CrossRef] [Green Version]
- Kurubanjerdjit, N. Identifying the regulation mechanism of phytochemicals on triple negative breast cancer’s biological network. Gene Rep. 2020, 19, 100656. [Google Scholar] [CrossRef]
- Malla, R.R.; Deepak, K.; Merchant, N.; Dasari, V.R. Breast Tumor Microenvironment: Emerging target of therapeutic phytochemicals. Phytomedicine 2020, 70, 153227. [Google Scholar] [CrossRef] [PubMed]
- Ossovskaya, V.; Wang, Y.; Budoff, A.; Xu, Q.; Lituev, A.; Potapova, O.; Vansant, G.; Monforte, J.; Daraselia, N. Exploring Molecular Pathways of Triple-Negative Breast Cancer. Genes Cancer 2011, 2, 870–879. [Google Scholar] [CrossRef]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Anders, C.K.; Zagar, T.M.; Carey, L.A. The management of early-stage and metastatic triple-negative breast cancer: A review. Hematol. Oncol. Clin. N. Am. 2013, 27, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Ensenyat-Mendez, M.; Llinàs-Arias, P.; Orozco, J.I.J.; Íñiguez-Muñoz, S.; Salomon, M.P.; Sesé, B.; DiNome, M.L.; Marzese, D.M. Current Triple-Negative Breast Cancer Subtypes: Dissecting the Most Aggressive Form of Breast Cancer. Front. Oncol. 2021, 11, 681476. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, B.D.; Jovanović, B.; Chen, X.; Estrada, M.V.; Johnson, K.N.; Shyr, Y.; Moses, H.L.; Sanders, M.E.; Pietenpol, J.A. Refinement of triple-negative breast cancer molecular subtypes: Implications for neoadjuvant chemotherapy selection. PLoS ONE 2016, 11, e0157368. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J. Pathol. 2014, 232, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Jhan, J.-R.; Andrechek, E.R. Triple-negative breast cancer and the potential for targeted therapy. Pharmacogenomics 2017, 18, 1595–1609. [Google Scholar] [CrossRef] [Green Version]
- He, J.; McLaughlin, R.P.; van der Noord, V.; Foekens, J.A.; Martens, J.W.; van Westen, G.; Zhang, Y.; van de Water, B. Multi-targeted kinase inhibition alleviates mTOR inhibitor resistance in triple-negative breast cancer. Breast Cancer Res. Treat. 2019, 178, 263–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Baggerly, K.A.; Wang, Y.; Zhang, Y.; Gonzalez-Angulo, A.M.; Meric-Bernstam, F.; Valero, V.; Lehmann, B.D.; Pietenpol, J.A.; Hortobagyi, G.N. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013, 19, 5533–5540. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, B.D.; Bauer, J.A.; Schafer, J.M.; Pendleton, C.S.; Tang, L.; Johnson, K.C.; Chen, X.; Balko, J.M.; Gómez, H.; Arteaga, C.L.; et al. PIK3CA mutations in androgen receptor-positive triple negative breast cancer confer sensitivity to the combination of PI3K and androgen receptor inhibitors. Breast Cancer Res. 2014, 16, 406. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Jézéquel, P.; Kerdraon, O.; Hondermarck, H.; Guérin-Charbonnel, C.; Lasla, H.; Gouraud, W.; Canon, J.L.; Gombos, A.; Dalenc, F.; Delaloge, S.; et al. Identification of three subtypes of triple-negative breast cancer with potential therapeutic implications. Breast Cancer Res. 2019, 21, 65. [Google Scholar] [CrossRef]
- Andre, F.; Zielinski, C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. 2012, 23, vi46–vi51. [Google Scholar] [CrossRef] [PubMed]
- Collignon, J.; Lousberg, L.; Schroeder, H.; Jerusalem, G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer 2016, 8, 93–107. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.; Gao, R.; Sei, E.; Brandt, R.; Hartman, J.; Hatschek, T.; Crosetto, N.; Foukakis, T.; Navin, N.E. Chemoresistance Evolution in Triple-Negative Breast Cancer Delineated by Single-Cell Sequencing. Cell 2018, 173, 879–893.e13. [Google Scholar] [CrossRef] [Green Version]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [Green Version]
- Soliman, H.; Khalil, F.; Antonia, S. PD-L1 expression is increased in a subset of basal type breast cancer cells. PLoS ONE 2014, 9, e88557. [Google Scholar] [CrossRef] [Green Version]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Price, T.J.; Townsend, A.R. Druggable molecular targets for the treatment of triple negative breast cancer. J. Breast Cancer 2019, 22, 341–361. [Google Scholar] [CrossRef] [Green Version]
- Selby, M.J.; Engelhardt, J.J.; Quigley, M.; Henning, K.A.; Chen, T.; Srinivasan, M.; Korman, A.J. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol. Res. 2013, 1, 32–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vargas, F.A.; Furness, A.J.S.; Litchfield, K.; Joshi, K.; Rosenthal, R.; Ghorani, E.; Solomon, I.; Lesko, M.H.; Ruef, N.; Roddie, C.; et al. Fc Effector Function Contributes to the Activity of Human Anti-CTLA-4 Antibodies. Cancer Cell 2018, 33, 649–663.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchbinder, E.I.; Desai, A. CTLA-4 and PD-1 pathways similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 2016, 39, 98. [Google Scholar] [CrossRef] [Green Version]
- Vikas, P.; Borcherding, N.; Zhang, W. The clinical promise of immunotherapy in triple-negative breast cancer. Cancer Manag. Res. 2018, 10, 6823–6833. [Google Scholar] [CrossRef] [Green Version]
- Qin, S.; Xu, L.; Yi, M.; Yu, S.; Wu, K.; Luo, S. Novel immune checkpoint targets: Moving beyond PD-1 and CTLA-4. Mol. Cancer 2019, 18, 155. [Google Scholar] [CrossRef]
- Harris, L.G.; Pannell, L.K.; Singh, S.; Samant, R.S.; Shevde, L.A. Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61. Oncogene 2012, 31, 3370–3380. [Google Scholar] [CrossRef] [Green Version]
- Colavito, S.A.; Zou, M.R.; Yan, Q.; Nguyen, D.X.; Stern, D.F. Significance of glioma-associated oncogene homolog 1 (GLI1) expression in claudin-low breast cancer and crosstalk with the nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) pathway. Breast Cancer Res. 2014, 16, 444. [Google Scholar] [CrossRef] [Green Version]
- Riobo, N.A.; Haines, G.M.; Emerson, C.P. Protein kinase C-δ and mitogen-activated protein/extracellular signal–regulated kinase-1 control GLI activation in Hedgehog signaling. Cancer Res. 2006, 66, 839–845. [Google Scholar] [CrossRef] [Green Version]
- Goel, H.L.; Pursell, B.; Chang, C.; Shaw, L.M.; Mao, J.; Simin, K.; Kumar, P.; Vander Kooi, C.W.; Shultz, L.D.; Greiner, D.L.; et al. GLI1 regulates a novel neuropilin-2/α6β1 integrin based autocrine pathway that contributes to breast cancer initiation. EMBO Mol. Med. 2013, 5, 488–508. [Google Scholar] [CrossRef]
- Duan, Z.H.; Wang, H.C.; Zhao, D.M.; Ji, X.X.; Song, M.; Yang, X.J.; Cui, W. Cooperatively transcriptional and epigenetic regulation of sonic hedgehog overexpression drives malignant potential of breast cancer. Cancer Sci. 2015, 106, 1084–1091. [Google Scholar] [CrossRef]
- Tacchini, L.; de Ponti, C.; Matteucci, E.; Follis, R.; Desiderio, M.A. Hepatocyte growth factor-activated NF-κB regulates HIF-1 activity and ODC expression, implicated in survival, differently in different carcinoma cell lines. Carcinogenesis 2004, 25, 2089–2100. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.; Wang, S.; Shen, Q.; Zhou, X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett. 2018, 415, 117–128. [Google Scholar] [CrossRef]
- Dhillon, K.K.; Swisher, E.M.; Taniguchi, T. Secondary mutations of BRCA1/2 and drug resistance. Cancer Sci. 2011, 102, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Catana, A.; Apostu, A.P.; Antemie, R.G. Multi gene panel testing for hereditary breast cancer—Is it ready to be used? Med. Pharm. Rep. 2019, 92, 220. [Google Scholar] [CrossRef]
- Couch, F.J.; Hart, S.N.; Sharma, P.; Toland, A.E.; Wang, X.; Miron, P.; Olson, J.E.; Godwin, A.K.; Pankratz, V.S.; Olswold, C.; et al. Inherited mutations in 17 breast cancer susceptibility genes among a large triple-negative breast cancer cohort unselected for family history of breast cancer. J. Clin. Oncol. 2015, 33, 304–311. [Google Scholar] [CrossRef]
- Kim, M.P.; Zhang, Y.; Lozano, G. Mutant p53: Multiple mechanisms define biologic activity in cancer. Front. Oncol. 2015, 5, 249. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, S.K.; Yap, T.A.; de Bono, J.S. The Emerging Role of Poly(ADP-Ribose) Polymerase Inhibitors in Cancer Treatment. Curr. Drug Targets 2011, 12, 2034–2044. [Google Scholar] [CrossRef]
- Davar, D.; Beumer, J.H.; Hamieh, L.; Tawbi, H. Role of PARP Inhibitors in Cancer Biology and Therapy. Curr. Med. Chem. 2012, 19, 3907–3921. [Google Scholar] [CrossRef] [PubMed]
- Livraghi, L.; Garber, J.E. PARP inhibitors in the management of breast cancer: Current data and future prospects. BMC Med. 2015, 13, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaufman, B.; Shapira-Frommer, R.; Schmutzler, R.K.; Audeh, M.W.; Friedlander, M.; Balmaña, J.; Mitchell, G.; Fried, G.; Stemmer, S.M.; Hubert, A.; et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 2015, 33, 244. [Google Scholar] [CrossRef]
- Pahuja, S.; Beumer, J.H.; Appleman, L.J.; Tawbi, H.A.-H.; Stoller, R.G.; Lee, J.J.; Lin, Y.; Ding, F.; Yu, J.; Belani, C.P.; et al. A phase I study of veliparib (ABT-888) in combination with weekly carboplatin and paclitaxel in advanced solid malignancies and enriched for triple-negative breast cancer (TNBC). J. Clin. Oncol. 2015, 33, 1015. [Google Scholar] [CrossRef]
- New, M.; Olzscha, H.; La Thangue, N.B. HDAC inhibitor-based therapies: Can we interpret the code? Mol. Oncol. 2012, 6, 637–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garmpis, N.; Damaskos, C.; Garmpi, A.; Kalampokas, E.; Kalampokas, T.; Spartalis, E.; Daskalopoulou, A.; Valsami, S.; Kontos, M.; Nonni, A. Histone deacetylases as new therapeutic targets in triple-negative breast cancer: Progress and promises. Cancer Genom. Proteom. 2017, 14, 299–313. [Google Scholar]
- Olzscha, H.; Sheikh, S.; La Thangue, N.B. Deacetylation of chromatin and gene expression regulation: A new target for epigenetic therapy. Crit. Rev. Oncog. 2015, 20, 1–17. [Google Scholar] [CrossRef]
- Tate, C.R.; Rhodes, L.V.; Segar, H.C.; Driver, J.L.; Pounder, F.N.; Burow, M.E.; Collins-Burow, B.M. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res. 2012, 14, R79. [Google Scholar] [CrossRef] [Green Version]
- Slingerland, M.; Guchelaar, H.J.; Gelderblom, H. Histone deacetylase inhibitors: An overview of the clinical studies in solid tumors. Anti-Cancer Drugs 2014, 25, 140–149. [Google Scholar] [CrossRef]
- Schech, A.; Kazi, A.; Yu, S.; Shah, P.; Sabnis, G. Histone deacetylase inhibitor entinostat inhibits tumor-initiating cells in triple-negative breast cancer cells. Mol. Cancer Ther. 2015, 14, 1848–1857. [Google Scholar] [CrossRef] [Green Version]
- Merino, V.F.; Nguyen, N.; Jin, K.; Sadik, H.; Cho, S.; Korangath, P.; Han, L.; Foster, Y.M.N.; Zhou, X.C.; Zhang, Z.; et al. Combined treatment with epigenetic, differentiating, and chemotherapeutic agents cooperatively targets tumor-initiating cells in triple-negative breast cancer. Cancer Res. 2016, 76, 2013–2024. [Google Scholar] [CrossRef] [Green Version]
- Kai, M.; Kanaya, N.; Wu, S.V.; Mendez, C.; Nguyen, D.; Luu, T.; Chen, S. Targeting breast cancer stem cells in triple-negative breast cancer using a combination of LBH589 and salinomycin. Breast Cancer Res. Treat. 2015, 151, 281–294. [Google Scholar] [CrossRef]
- Mrklić, I.; Pogorelić, Z.; Ćapkun, V.; Tomić, S.Ž. Expression of androgen receptors in triple negative breast carcinomas. Acta Histochem. 2013, 115, 344–348. [Google Scholar] [CrossRef]
- Astvatsaturyan, K.; Yue, Y.; Walts, A.E.; Bose, S. Androgen receptor positive triple negative breast cancer: Clinicopathologic, prognostic, and predictive features. PLoS ONE 2018, 13, e0197827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietri, E.; Conteduca, V.; Andreis, D.; Massa, I.; Melegari, E.; Sarti, S.; Cecconetto, L.; Schirone, A.; Bravaccini, S.; Serra, P.; et al. Androgen receptor signaling pathways as a target for breast cancer treatment. Endocr. Relat. Cancer 2016, 23, R485–R498. [Google Scholar] [CrossRef] [Green Version]
- Gerratana, L.; Basile, D.; Buono, G.; de Placido, S.; Giuliano, M.; Minichillo, S.; Coinu, A.; Martorana, F.; de Santo, I.; Del Mastro, L.; et al. Androgen receptor in triple negative breast cancer: A potential target for the targetless subtype. Cancer Treat. Rev. 2018, 68, 102–110. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, R.; Yuan, Z.; Wang, S.; Peng, J.; Lin, G.; Jiang, X.; Qin, T. Prognostic value of androgen receptor expression in operable triple-negative breast cancer: A retrospective analysis based on a tissue microarray. Med. Oncol. 2012, 29, 406–410. [Google Scholar] [CrossRef]
- Caiazza, F.; Murray, A.; Madden, S.F.; Synnott, N.C.; Ryan, E.J.; O’Donovan, N.; Crown, J.; Duffy, M.J. Preclinical evaluation of the AR inhibitor enzalutamide in triple-negative breast cancer cells. Endocr. Relat. Cancer 2016, 23, 323–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asano, Y.; Kashiwagi, S.; Goto, W.; Tanaka, S.; Morisaki, T.; Takashima, T.; Noda, S.; Onoda, N.; Ohsawa, M.; Hirakawa, K. Expression and clinical significance of androgen receptor in triple-negative breast cancer. Cancers 2017, 9, 4. [Google Scholar] [CrossRef] [Green Version]
- Hu, R.; Dawood, S.; Holmes, M.D.; Collins, L.C.; Schnitt, S.J.; Cole, K.; Marotti, J.D.; Hankinson, S.E.; Colditz, G.A.; Tamimi, R.M. Androgen receptor expression and breast cancer survival in postmenopausal women. Clin. Cancer Res. 2011, 17, 1867–1874. [Google Scholar] [CrossRef] [Green Version]
- Rahim, B.; O’Regan, R. AR signaling in breast cancer. Cancers 2017, 9, 21. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.; Jae, E.; Yoon, M. Influence of androgen receptor expression on the survival outcomes in breast cancer: A meta-analysis. J. Breast Cancer 2015, 18, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic breast cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef] [Green Version]
- Barton, V.N.; D’Amato, N.C.; Gordon, M.A.; Lind, H.T.; Spoelstra, N.S.; Babbs, B.L.; Heinz, R.E.; Elias, A.; Jedlicka, P.; Jacobsen, B.M.; et al. Multiple molecular subtypes of triple-negative breast cancer critically rely on androgen receptor and respond to enzalutamide in vivo. Mol. Cancer Ther. 2015, 14, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Traina, T.A.; Miller, K.; Yardley, D.A.; Eakle, J.; Schwartzberg, L.S.; O’Shaughnessy, J.; Gradishar, W.; Schmid, P.; Winer, E.; Kelly, C.; et al. Enzalutamide for the treatment of androgen receptor-expressing triple-negative breast cancer. J. Clin. Oncol. 2018, 36, 884–890. [Google Scholar] [CrossRef]
- Bardia, A.; Gucalp, A.; DaCosta, N.; Gabrail, N.; Danso, M.; Ali, H.; Blackwell, K.L.; Carey, L.A.; Eisner, J.R.; Baskin-Bey, E.S.; et al. Phase 1 study of seviteronel, a selective CYP17 lyase and androgen receptor inhibitor, in women with estrogen receptor-positive or triple-negative breast cancer. Breast Cancer Res. Treat. 2018, 171, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Crouch, B.T.; Gallagher, J.; Wang, R.; Duer, J.; Hall, A.; Soo, M.S.; Hughes, P.; Haystead, T.; Ramanujam, N. Exploiting heat shock protein expression to develop a non-invasive diagnostic tool for breast cancer. Sci. Rep. 2019, 9, 3461. [Google Scholar] [CrossRef] [PubMed]
- Den, R.B.; Lu, B. Heat shock protein 90 inhibition: Rationale and clinical potential. Ther. Adv. Med. Oncol. 2012, 4, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Caldas-Lopes, E.; Cerchietti, L.; Ahn, J.H.; Clement, C.C.; Robles, A.I.; Rodina, A.; Moulick, K.; Taldone, T.; Gozrnan, A.; Guo, Y.; et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc. Natl. Acad. Sci. USA 2009, 106, 8368–8373. [Google Scholar] [CrossRef] [Green Version]
- Proverbs-Singh, T.; Feldman, J.L.; Morris, M.J.; Autio, K.A.; Traina, T.A. Targeting the androgen receptor in prostate and breast cancer: Several new agents in development. Endocr. Relat. Cancer 2015, 22, R87–R106. [Google Scholar] [CrossRef] [Green Version]
- Jhaveri, K.; Chandarlapaty, S.; Lake, D.; Gilewski, T.; Robson, M.; Goldfarb, S.; Drullinsky, P.; Sugarman, S.; Wasserheit-Leiblich, C.; Fasano, J.; et al. A phase II open-label study of ganetespib, a novel heat shock protein 90 inhibitor for patients with Metastatic breast cancer. Clin. Breast Cancer 2014, 14, 154–160. [Google Scholar] [CrossRef]
- Kou, X.; Jiang, X.; Liu, H.; Wang, X.; Sun, F.; Han, J.; Fan, J.; Feng, G.; Lin, Z.; Jiang, L.; et al. Simvastatin functions as a heat shock protein 90 inhibitor against triple-negative breast cancer. Cancer Sci. 2018, 109, 3272–3284. [Google Scholar] [CrossRef]
- Mitri, Z.; Karakas, C.; Wei, C.; Briones, B.; Simmons, H.; Ibrahim, N.; Alvarez, R.; Murray, J.L.; Keyomarsi, K.; Moulder, S. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Invest. New Drugs 2015, 33, 890–894. [Google Scholar] [CrossRef]
- Chien, A.J.; Rahmaputri, S.; Dittrich, H.F.; Majure, M.C.; Rugo, H.S.; Melisko, M.E.; Goga, A. A phase Ib trial of the cyclin-dependent kinase inhibitor dinaciclib (dina) in combination with pembrolizumab (P) in patients with advanced triple-negative breast cancer (TNBC). J. Clin. Oncol. 2019, 13, 1072. [Google Scholar] [CrossRef]
- Paplomata, E.; O’regan, R. The PI3K/AKT/mTOR pathway in breast cancer: Targets, trials and biomarkers. Ther. Adv. Med. Oncol. 2014, 6, 154–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.J.; Tan, T.J.Y.; Dent, R.A. Novel therapeutic avenues in triple-negative breast cancer: PI3K/AKT inhibition, androgen receptor blockade, and beyond. Ther. Adv. Med. Oncol. 2019, 11. [Google Scholar] [CrossRef]
- Sharma, V.; Sharma, A.K.; Punj, V.; Priya, P. Recent nanotechnological interventions targeting PI3K/Akt/mTOR pathway: A focus on breast cancer. Semin. Cancer Biol. 2019, 59, 133–146. [Google Scholar] [CrossRef]
- van der Noord, V.E.; McLaughlin, R.P.; Smid, M.; Foekens, J.A.; Martens, J.W.M.; Zhang, Y.; van de Water, B. An increased cell cycle gene network determines MEK and Akt inhibitor double resistance in triple-negative breast cancer. Sci. Rep. 2019, 9, 13308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, G.L.; Amos, K.D.; Duncan, J.S.; Whittle, M.; Zawistowki, J.; Goulet, D.; He, X.; Noe, J.; Perou, C.M.; Earp, S.; et al. Kinome reprogramming response to MEK inhibition: A window-of-opportunity trial in triple-negative breast cancer (TNBC). J. Clin. Oncol. 2013, 31, 512. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Mrozek, E.; Lustberg, M.; Wesolowski, R.; Layman, R.; Abdel-Rasoul, M.; Timmers, C.; Patrick, R.; Sexton, J.; Macrae, E.; et al. Abstract LB-216: NCI 9455: Phase II study of trametinib followed by trametinib plus AKT inhibitor, GSK2141795 in patients with advanced triple negative breast cancer. In Proceedings of the AACR 107th Annual Meeting, New Orleans, LA, USA, 16–20 April 2016. [Google Scholar] [CrossRef]
- Shuai, K.; Liu, B. Regulation of JAK–STAT signalling in the immune system. Nat. Rev. Immunol. 2003, 3, 900–911. [Google Scholar] [CrossRef]
- Balko, J.M.; Schwarz, L.J.; Luo, N.; Estrada, M.V.; Giltnane, J.M.; Dávila-González, D.; Wang, K.; Sánchez, V.; Dean, P.T.; Combs, S.E.; et al. Triple-negative breast cancers with amplification of JAK2 at the 9p24 locus demonstrate JAK2-specific dependence. Sci. Transl. Med. 2016, 8, 334ra53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barrett, M.T.; Anderson, K.S.; Lenkiewicz, E.; Andreozzi, M.; Cunliffe, H.E.; Klassen, C.L.; Dueck, A.C.; McCullough, A.E.; Reddy, S.K.; Ramanathan, R.K.; et al. Genomic amplification of 9p24.1 targeting JAK2, PD-L1, and PD-L2 is enriched in high-risk triple negative breast cancer. Oncotarget 2015, 6, 26483. [Google Scholar] [CrossRef] [Green Version]
- Stanek, L.; Tesarova, P.; Vocka, M.; Petruzelka, L. Analysis of the JAK2 gene in triple-negative breast cancer (TNBC). Ann. Oncol. 2018, 29, viii95. [Google Scholar] [CrossRef]
- Stover, D.G.; Gil Del Alcazar, C.R.; Brock, J.; Guo, H.; Overmoyer, B.; Balko, J.; Xu, Q.; Bardia, A.; Tolaney, S.M.; Gelman, R.; et al. Phase II study of ruxolitinib, a selective JAK1/2 inhibitor, in patients with metastatic triple-negative breast cancer. NPJ Breast Cancer 2018, 4, 10. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef] [PubMed]
- Huynh, J.; Chand, A.; Gough, D.; Ernst, M. Therapeutically exploiting STAT3 activity in cancer—Using tissue repair as a road map. Nat. Rev. Cancer 2019, 19, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.J.; Yan, L.; Zhang, J.; Zhang, W.D. STAT3 as a potential therapeutic target in triple negative breast cancer: A systematic review. J. Exp. Clin. Cancer Res. 2019, 38, 195. [Google Scholar] [CrossRef] [PubMed]
- Morris, P.G.; Rota, S.; Cadoo, K.; Zamora, S.; Patil, S.; D’Andrea, G.; Gilewski, T.; Bromberg, J.; Dang, C.; Dickler, M. Phase II study of paclitaxel and dasatinib in metastatic breast cancer. Clin. Breast Cancer 2018, 18, 387–394. [Google Scholar] [CrossRef]
- Furtek, S.L.; Backos, D.S.; Matheson, C.J.; Reigan, P. Strategies and Approaches of Targeting STAT3 for Cancer Treatment. ACS Chem. Biol. 2016, 11, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Xiong, A.; Yang, Z.; Shen, Y.; Zhou, J.; Shen, Q. Transcription factor STAT3 as a novel molecular target for cancer prevention. Cancers 2014, 6, 926–957. [Google Scholar] [CrossRef] [Green Version]
- Park, S.-Y.; Choi, J.-H.; Nam, J.-S. Targeting cancer stem cells in triple-negative breast cancer. Cancers 2019, 11, 965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takebe, N.; Miele, L.; Harris, P.J.; Jeong, W.; Bando, H.; Kahn, M.; Yang, S.X.; Ivy, S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: Clinical update. Nat. Rev. Clin. Oncol. 2015, 12, 445–464. [Google Scholar] [CrossRef]
- Solzak, J.P.; Atale, R.V.; Hancock, B.A.; Sinn, A.L.; Pollok, K.E.; Jones, D.R.; Radovich, M. Dual PI3K and Wnt pathway inhibition is a synergistic combination against triple negative breast cancer. NPJ Breast Cancer 2017, 3, 17. [Google Scholar] [CrossRef]
- Liu, J.; Pan, S.; Hsieh, M.H.; Ng, N.; Sun, F.; Wang, T.; Kasibhatla, S.; Schuller, A.G.; Li, A.G.; Cheng, D.; et al. Targeting Wnt-driven cancer through the inhibition of Porcupine by LGK974. Proc. Natl. Acad. Sci. USA 2013, 110, 20224–20229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, G.B.; Hong, I.S.; Kim, R.J.; Lee, S.Y.; Park, S.J.; Lee, E.S.; Park, J.H.; Yun, C.H.; Chung, J.U.; Lee, K.J.; et al. Wnt/β-catenin small-molecule inhibitor CWP232228 preferentially inhibits the growth of breast cancer stem-like cells. Cancer Res. 2015, 75, 1691–1702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurney, A.; Axelrod, F.; Bond, C.J.; Cain, J.; Chartier, C.; Donigan, L.; Fischer, M.; Chaudhari, A.; Ji, M.; Kapoun, A.M.; et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 11717–11722. [Google Scholar] [CrossRef] [Green Version]
- Fischer, M.M.; Yen, W.-C.; Zheng, C.; Henner, R.; Cattaruzza, F.; Tang, T.; Yeung, P.; Biswas, T.; Lewicki, J.; Gurney, A.; et al. Abstract 4233: Wnt pathway antagonist ipafricept (FZD8-Fc, OMP-54F28) inhibits tumor growth and reduces tumor-initiating cell frequency in ovarian patient-derived xenograft models. Cancer Res. 2015, 75, 4233. [Google Scholar] [CrossRef]
- Blagodatski, A.; Poteryaev, D.; Katanaev, V.L. Targeting the Wnt pathways for therapies. Mol. Cell. Ther. 2014, 2, 28. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.W.; Xu, J.; Zhu, G.Y.; Huang, Z.J.; Lu, Y.; Li, X.Q.; Wang, N.; Zhang, F.X. Apigenin suppresses the stem cell-like properties of triple-negative breast cancer cells by inhibiting YAP/TAZ activity. Cell Death Discov. 2018, 4, 105. [Google Scholar] [CrossRef]
- Curigliano, G.; Pivot, X.; Cortés, J.; Elias, A.; Cesari, R.; Khosravan, R.; Collier, M.; Huang, X.; Cataruozolo, P.E.; Kern, K.A.; et al. Randomized phase II study of sunitinib versus standard of care forpatients with previously treated advanced triple-negative breastcancer. Breast 2013, 22, 650–656. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Nico, B.; Ruggieri, S.; Tamma, R.; Simone, G.; Mangia, A. Angiogenesis and antiangiogenesis in triple-negative breast cancer. Transl. Oncol. 2016, 9, 453–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, R.J.; Mac Gabhann, F. Expression of VEGF and Semaphorin Genes Define Subgroups of Triple Negative Breast Cancer. PLoS ONE 2013, 8, e61788. [Google Scholar] [CrossRef] [Green Version]
- Bahnassy, A.; Mohanad, M.; Ismail, M.F.; Shaarawy, S.; El-Bastawisy, A.; Zekri, A.R.N. Molecular biomarkers for prediction of response to treatment and survival in triple negative breast cancer patients from Egypt. Exp. Mol. Pathol. 2015, 99, 303–311. [Google Scholar] [CrossRef]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtiö, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef] [PubMed]
- Changavi, A.A.; Shashikala, A.; Ramji, A.S. Epidermal Growth Factor Receptor Expression in Triple Negative and Nontriple Negative Breast Carcinomas. J. Lab. Physicians 2015, 7, 79–83. [Google Scholar] [CrossRef] [PubMed]
- Rimawi, M.F.; Shetty, P.B.; Weiss, H.L.; Schiff, R.; Osborne, C.K.; Chamness, G.C.; Elledge, R.M. Epidermal growth factor receptor expression in breast cancer association with biologic phenotype and clinical outcomes. Cancer 2010, 116, 1234–1242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, H.; Zhang, D.; Bartholomeusz, C.; Doihara, H.; Hortobagyi, G.N.; Ueno, N.T. Role of epidermal growth factor receptor in breast cancer. Breast Cancer Res. Treat. 2012, 136, 331–345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cetin, I.; Topcul, M. Triple negative breast cancer. Asian Pac. J. Cancer Prev. 2014, 15, 2427–2431. [Google Scholar] [CrossRef] [Green Version]
- Zakaria, Z.; Zulkifle, M.F.; Hasan, W.A.N.W.; Azhari, A.K.; Raub, S.H.A.; Eswaran, J.; Soundararajan, M.; Syed Husain, S.N.A. Epidermal growth factor receptor (EGFR) gene alteration and protein overexpression in Malaysian triple-negative breast cancer (TNBC) cohort. Onco Targets Ther. 2019, 12, 7749–7756. [Google Scholar] [CrossRef] [Green Version]
- Sharpe, R.; Pearson, A.; Herrera-Abreu, M.T.; Johnson, D.; Mackay, A.; Welti, J.C.; Natrajan, R.; Reynolds, A.R.; Reis-Filho, J.S.; Ashworth, A.; et al. FGFR signaling promotes the growth of triple-negative and basal-like breast cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 2011, 17, 5275–5286. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dienstmann, R.; Rodon, J.; Prat, A.; Perez-Garcia, J.; Adamo, B.; Felip, E.; Cortes, J.; Iafrate, A.; Nuciforo, P.; Tabernero, J. Genomic aberrations in the FGFR pathway: Opportunities for targeted therapies in solid tumors. Ann. Oncol. 2014, 25, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Perez-Garcia, J.; Muñoz-Couselo, E.; Soberino, J.; Racca, F.; Cortes, J. Targeting FGFR pathway in breast cancer. Breast 2018, 37, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-Y.; Yi, J.Y.; Kim, M.-H.; Song, K.-H.; Kang, S.-M.; Ahn, J.; Hwang, S.-G.; Nam, K.-Y.; Song, J.-Y. IM-412 inhibits the invasion of human breast carcinoma cells by blocking FGFR-mediated signaling. Oncol. Rep. 2015, 34, 2731–2737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldenberg, D.M.; Cardillo, T.M.; Govindan, S.V.; Rossi, E.A.; Sharkey, R.M. Trop-2 is a novel target for solid cancer therapy with sacituzumab govitecan (IMMU-132), an antibody-drug conjugate (ADC). Oncotarget 2015, 6, 22496. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, D.M.; Stein, R.; Sharkey, R.M. The emergence of trophoblast cell-surface antigen 2 (TROP-2) as a novel cancer target. Oncotarget 2018, 9, 28989–29006. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, R.M.; McBride, W.J.; Cardillo, T.M.; Govindan, S.V.; Wang, Y.; Rossi, E.A.; Chang, C.H.; Goldenberg, D.M. Enhanced delivery of SN-38 to human tumor xenografts with an anti-Trop-2-SN-38 antibody conjugate (sacituzumab govitecan). Clin. Cancer Res. 2015, 21, 5131–5138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starodub, A.N.; Ocean, A.J.; Shah, M.A.; Guarino, M.J.; Picozzi, V.J.; Vahdat, L.T.; Thomas, S.S.; Govindan, S.V.; Maliakal, P.P.; Wegener, W.A.; et al. First-in-human trial of a novel anti-trop-2 Antibody-SN-38 conjugate, sacituzumab govitecan, for the treatment of diverse metastatic solid tumors. Clin. Cancer Res. 2015, 21, 3870–3878. [Google Scholar] [CrossRef] [Green Version]
- Bendell, J.; Saleh, M.; Rose, A.A.N.; Siegel, P.M.; Hart, L.; Sirpal, S.; Jones, S.; Green, J.; Crowley, E.; Simantov, R.; et al. Phase I/II study of the antibody-drug conjugate glembatumumab vedotin in patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2014, 32, 3619–3625. [Google Scholar] [CrossRef]
- Yardley, D.A.; Weaver, R.; Melisko, M.E.; Saleh, M.N.; Arena, F.P.; Forero, A.; Cigler, T.; Stopeck, A.; Citrin, D.; Oliff, I.; et al. EMERGE: A randomized phase II study of the antibody-drug conjugate glembatumumab vedotin in advanced glycoprotein NMB - Expressing breast cancer. J. Clin. Oncol. 2015, 33, 1609–1619. [Google Scholar] [CrossRef]
- Marquez-Nostra, B.V.; Lee, S.; Laforest, R.; Vitale, L.; Nie, X.; Hyrc, K.; Keler, T.; Hawthorne, T.; Hoog, J.; Li, S.; et al. Preclinical PET imaging of glycoprotein non-metastatic melanoma B in triple negative breast cancer: Feasibility of an antibody-based companion diagnostic agent. Oncotarget 2017, 8, 104303–104314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liston, D.R.; Davis, M. Clinically relevant concentrations of anticancer drugs: A guide for nonclinical studies. Clin. Cancer Res. 2017, 23, 3489–3498. [Google Scholar] [CrossRef] [Green Version]
- Lichota, A.; Gwozdzinski, K. Anticancer activity of natural compounds from plant and marine environment. Int. J. Mol. Sci. 2018, 19, 3533. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Liu, Z.J.; Chen, T.; Zhao, D. Pharmaceutical properties of calycosin, the major bioactive isoflavonoid in the dry root extract of Radix astragali. Pharm. Biol. 2014, 52, 1217–1222. [Google Scholar] [CrossRef] [Green Version]
- Massi, A.; Bortolini, O.; Ragno, D.; Bernardi, T.; Sacchetti, G.; Tacchini, M.; de Risi, C. Research progress in the modification of quercetin leading to anticancer agents. Molecules 2017, 22, 1270. [Google Scholar] [CrossRef]
- Kim, J.Y.; Dao, T.T.P.; Song, K.; Park, S.B.; Jang, H.; Park, M.K.; Gan, S.U.; Kim, Y.S. Annona muricata Leaf Extract Triggered Intrinsic Apoptotic Pathway to Attenuate Cancerous Features of Triple Negative Breast Cancer MDA-MB-231 Cells. Evid. Based Complement. Alternat. Med. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Shi, R.; Wang, X.; Shen, H.-M. Luteolin, a flavonoid with potential for cancer prevention and therapy. Curr. Cancer Drug Targets 2008, 8, 634–646. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.; Kuang, G.; Wan, J.; Zhang, X.; Li, H.; Gong, X.; Li, H. Luteolin suppresses the metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via downregulation of β-catenin expression. Oncol. Rep. 2017, 37, 895–902. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.T.; Liang, Y.; Besch-Williford, C.; Hyder, S.M. Luteolin inhibits lung metastasis, cell migration, and viability of triple-negative breast cancer cells. Breast Cancer 2016, 9, 9–19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, K.J.; Tsai, H.Y.; Tsai, C.C.; Chen, T.Y.; Hsieh, T.H.; Chen, C.L.; Mbuyisa, L.; Huang, Y.B.; Lin, M.W. Luteolin Inhibits Breast Cancer Stemness and Enhances Chemosensitivity through the Nrf2-Mediated Pathway. Molecules 2021, 26, 6452. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, M.Y.; Hashim, N.M.; Mariod, A.A.; Mohan, S.; Abdulla, M.A.; Abdelwahab, S.I.; Arbab, I.A. α-Mangostin from Garcinia mangostana Linn: An updated review of its pharmacological properties. Arab. J. Chem. 2016, 9, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Scolamiero, G.; Pazzini, C.; Bonafè, F.; Guarnieri, C.; Muscari, C. Effects of α-mangostin on viability, growth and cohesion of multicellular spheroids derived from human breast cancer cell lines. Int. J. Med. Sci. 2018, 15, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Li, J.; Ning, H.; Yuan, Z.; Zhong, Y.; Wu, S.; Zeng, J.Z. α-Mangostin Induces Apoptosis and Inhibits Metastasis of Breast Cancer Cells via Regulating RXRα-AKT Signaling Pathway. Front. Pharmacol. 2021, 12, 739658. [Google Scholar] [CrossRef]
- Meghwal, M.; Goswami, T. Piper nigrum and piperine: An update. Phytother. Res. 2013, 27, 1121–1130. [Google Scholar] [CrossRef]
- Greenshields, A.L.; Doucette, C.D.; Sutton, K.M.; Madera, L.; Annan, H.; Yaffe, P.B.; Knickle, A.F.; Dong, Z.; Hoskin, D.W. Piperine inhibits the growth and motility of triple-negative breast cancer cells. Cancer Lett. 2015, 357, 129–140. [Google Scholar] [CrossRef]
- Byun, H.J.; Darvin, P.; Kang, D.Y.; Sp, N.; Joung, Y.H.; Park, J.H.; Kim, S.J.; Yang, Y.M. Silibinin downregulates MMP2 expression via Jak2/STAT3 pathway and inhibits the migration and invasive potential in MDA-MB-231 cells. Oncol. Rep. 2017, 37, 3270–3278. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Venditti, A.; Sharifi-Rad, M.; Kręgiel, D.; Sharifi-Rad, J.; Durazzo, A.; Lucarini, M.; Santini, A.; Souto, E.B.; Novellino, E. The therapeutic potential of apigenin. Int. J. Mol. Sci. 2019, 20, 1305. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.; Mazzio, E.; Hilliard, A.; Oriaku, E.T.; Soliman, K.F.A. Effect of apigenin on whole transcriptome profile of TNFα-activated MDA-MB-468 triple negative breast cancer cells. Oncol. Lett. 2020, 19, 2123–2132. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Lee, Y.-H.; Sharma, A.R.; Park, J.-B.; Jagga, S.; Sharma, G.; Lee, S.-S.; Nam, J.-S. Quercetin induces apoptosis and cell cycle arrest in triple-negative breast cancer cells through modulation of Foxo3a activity. Korean J. Physiol. Pharmacol. 2017, 21, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Srinivasan, A.; Thangavel, C.; Liu, Y.; Shoyele, S.; Den, R.B.; Selvakumar, P.; Lakshmikuttyamma, A. Quercetin regulates β-catenin signaling and reduces the migration of triple negative breast cancer. Mol. Carcinog. 2016, 55, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.; Khalil, M.; Abdelghany, B.; Alkhuriji, A.; Sadek, O. Quercetin induces apoptosis in triple-negative breast cancer cells via inhibiting fatty acid synthase and ß-catenin. Int. J. Clin. Exp. Pathol. 2017, 10, 156–172. [Google Scholar]
- Khan, N.; Syed, D.N.; Ahmad, N.; Mukhtar, H. Fisetin: A dietary antioxidant for health promotion. Antioxid. Redox Signal. 2013, 19, 151–162. [Google Scholar] [CrossRef]
- Li, J.; Gong, X.; Jiang, R.; Lin, D.; Zhou, T.; Zhang, A.; Li, H.; Zhang, X.; Wan, J.; Kuang, G. Fisetin inhibited growth and metastasis of triple-negative breast cancer by reversing epithelial-to-mesenchymal transition via PTEN/Akt/GSK3β signal pathway. Front. Pharmacol. 2018, 9, 772. [Google Scholar] [CrossRef]
- Liang, Z.-J.; Wan, Y.; Zhu, D.-D.; Wang, M.-X.; Jiang, H.-M.; Huang, D.-L.; Luo, L.-F.; Chen, M.-J.; Yang, W.-P.; Li, H.-M. Resveratrol Mediates the Apoptosis of Triple Negative Breast Cancer Cells by Reducing POLD1 Expression. Front. Oncol. 2021, 11, 569295. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.D.; Sun, Y.; Zhou, W.J.; Xie, X.Z.; Zhou, Q.M.; Lu, Y.Y.; Su, S.B. Resveratrol Enhances Inhibition Effects of Cisplatin on Cell Migration and Invasion and Tumor Growth in Breast Cancer MDA-MB-231 Cell Models In Vivo and In Vitro. Molecules 2021, 26, 2204. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Tuorkey, M.J.; Thakral, F.; Sak, K.; Kumar, M.; Sharma, A.K.; Sharma, U.; Jain, A.; Aggarwal, V.; Bishayee, A. Molecular mechanisms of action of genistein in cancer: Recent advances. Front. Pharmacol. 2019, 10, 1336. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.; Zhou, W.; He, W.; Liu, X.; Ding, Q.; Ling, L.; Zha, X.; Wang, S. Genistein inhibits MDA-MB-231 triple-negative breast cancer cell growth by inhibiting NF-κB activity via the Notch-1 pathway. Int. J. Mol. Med. 2012, 30, 337–343. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Zhang, Q.; Wang, X.; Yang, X.; Wang, X.; Huang, Z.; Jiao, Y.; Wang, J. Quantitative phosphoproteomics reveals genistein as a modulator of cell cycle and DNA damage response pathways in triple-negative breast cancer cells. Int. J. Oncol. 2016, 48, 1016–1028. [Google Scholar] [CrossRef] [Green Version]
- Martin, A.C.B.; Fuzer, A.M.; Becceneri, A.B.; da Silva, J.A.; Tomasin, R.; Denoyer, D.; Kim, S.-H.; McIntyre, K.A.; Pearson, H.B.; Yeo, B. [10]-gingerol induces apoptosis and inhibits metastatic dissemination of triple negative breast cancer in vivo. Oncotarget 2017, 8, 72260. [Google Scholar] [CrossRef] [Green Version]
- Joo, J.H.; Hong, S.S.; Cho, Y.R.; Seo, D.W. 10-Gingerol inhibits proliferation and invasion of MDA-MB-231 breast cancer cells through suppression of Akt and p38MAPK activity. Oncol. Rep. 2016, 35, 779–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozmer, Z.; Perjési, P. Naturally occurring chalcones and their biological activities. Phytochem. Rev. 2016, 15, 87–120. [Google Scholar] [CrossRef]
- Muchtaridi, M.; Syahidah, H.N.; Subarnas, A.; Yusuf, M.; Bryant, S.D.; Langer, T. Molecular Docking and 3D-Pharmacophore Modeling to Study the Interactions of Chalcone Derivatives with Estrogen Receptor Alpha. Pharmaceuticals 2017, 10, 81. [Google Scholar] [CrossRef] [Green Version]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahya, A.; Akram, M.; Iqbal, M.; Docea, A.O. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2020, 11, 2068. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.; Jeengar, M.K.; Thummuri, D.; Koval, A.; Katanaev, V.L.; Marepally, S.; Naidu, V. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/β-catenin signaling cascades and reversal of epithelial-mesenchymal transition. BioFactors 2017, 43, 152–169. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Tan, H.-Y.; Li, L.; Yuen, M.-F.; Feng, Y. Berberine and Coptidis Rhizoma as potential anticancer agents: Recent updates and future perspectives. J. Ethnopharmacol. 2015, 176, 35–48. [Google Scholar] [CrossRef]
- Liu, D.; Meng, X.; Wu, D.; Qiu, Z.; Luo, H. A natural isoquinoline alkaloid with antitumor activity: Studies of the biological activities of berberine. Front. Pharmacol. 2019, 10, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khalki, L.; Maire, V.; Dubois, T.; Zyad, A. Berberine impairs the survival of triple negative breast cancer cells: Cellular and molecular analyses. Molecules 2020, 25, 506. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.-D.; Liu, X.-E.; Huang, D.-S. Curcumin induces apoptosis of triple-negative breast cancer cells by inhibition of EGFR expression. Mol. Med. Rep. 2012, 6, 1267–1270. [Google Scholar] [CrossRef] [Green Version]
- Zhou, X.; Jiao, D.; Dou, M.; Zhang, W.; Lv, L.; Chen, J.; Li, L.; Wang, L.; Han, X. Curcumin inhibits the growth of triple-negative breast cancer cells by silencing EZH2 and restoring DLC1 expression. J. Cell. Mol. Med. 2020, 24, 10648–10662. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.-C.; Sayseng, J.O.; Tu, S.-H.; Juan, T.-C.; Fang, C.-L.; Liao, Y.-C.; Chu, C.-Y.; Chang, H.-W.; Yen, Y.; Chen, L.; et al. Curcumin-induced antitumor effects on triple-negative breast cancer patient-derived xenograft tumor mice through inhibiting salt-induced kinase-3 protein. J. Food Drug Anal. 2021, 29, 622–637. [Google Scholar] [CrossRef] [PubMed]
- Braicu, C.; Gherman, C. Epigallocatechin gallate induce cell death and apoptosis in triple negative breast cancer cells Hs578T. J. Drug Target. 2013, 21, 250–256. [Google Scholar] [CrossRef]
- Zhu, J.; Jiang, Y.; Yang, X.; Wang, S.; Xie, C.; Li, X.; Li, Y.; Chen, Y.; Wang, X.; Meng, Y.; et al. Wnt/β-catenin pathway mediates (-)-Epigallocatechin-3-gallate (EGCG) inhibition of lung cancer stem cells. Biochem. Biophys. Res. Commun. 2017, 482, 15–21. [Google Scholar] [CrossRef]
- Oh, S.; Gwak, J.; Park, S.; Yang, C.S. Green tea polyphenol EGCG suppresses Wnt/β-catenin signaling by promoting GSK-3β- and PP2A-independent β-catenin phosphorylation/degradation. BioFactors 2014, 40, 586–595. [Google Scholar] [CrossRef] [PubMed]
- Hong, O.Y.; Noh, E.M.; Jang, H.Y.; Lee, Y.R.; Lee, B.K.; Jung, S.H.; Kim, J.S.; Youn, H.J. Epigallocatechin gallate inhibits the growth of MDA-MB-231 breast cancer cells via inactivation of the β-catenin signaling pathway. Oncol. Lett. 2017, 14, 441–446. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crous-Masó, J.; Palomeras, S.; Relat, J.; Camó, C.; Martínez-Garza, Ú.; Planas, M.; Feliu, L.; Puig, T. (-)-Epigallocatechin 3-Gallate Synthetic Analogues Inhibit Fatty Acid Synthase and Show Anticancer Activity in Triple Negative Breast Cancer. Molecules 2018, 23, 1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Li, H.; Yang, S.; Ma, W.; Liu, M.; Guo, S.; Zhan, J.; Zhang, H.; Tsang, S.Y.; Zhang, Z. Cyanidin-3-o-glucoside directly binds to ERα36 and inhibits EGFR-positive triple-negative breast cancer. Oncotarget 2016, 7, 68864. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Chen, L.; Ding, D.; Li, Z.; Cheng, L.; You, Q.; Zhang, S. Cyanidin-3-O-glucoside inhibits the β-catenin/MGMT pathway by upregulating miR-214-5p to reverse chemotherapy resistance in glioma cells. Sci. Rep. 2022, 12, 7773. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhao, B.; Liang, Q.; Zhang, Y.; Cai, J.; Li, G. The selective effect of glycyrrhizin and glycyrrhetinic acid on topoisomerase IIα and apoptosis in combination with etoposide on triple negative breast cancer MDA-MB-231 cells. Eur. J. Pharmacol. 2017, 809, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.C.; Chu, P.Y.; Liao, W.T.; Wu, M.Y.; Tsui, K.H.; Lin, L.T.; Huang, C.H.; Chen, L.L.; Li, C.J. Glycyrrhizic acid induces human MDA-MB-231 breast cancer cell death and autophagy via the ROS-mitochondrial pathway. Oncol. Rep. 2018, 39, 703–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Fang, H.; Wu, Q.; Wang, X.; Liu, R.; Li, F.; Xiao, J.; Yuan, L.; Zhou, Z.; Ma, J.; et al. Ilamycin E, a natural product of marine actinomycete, inhibits triple-negative breast cancer partially through ER stress-CHOP-Bcl-2. Int. J. Biol. Sci. 2019, 15, 1723–1732. [Google Scholar] [CrossRef]
- Xu, X.; Rajamanicham, V.; Xu, S.; Liu, Z.; Yan, T.; Liang, G.; Guo, G.; Zhou, H.; Wang, Y. Schisandrin A inhibits triple negative breast cancer cells by regulating Wnt/ER stress signaling pathway. Biomed. Pharmacother. 2019, 115, 108922. [Google Scholar] [CrossRef]
- Tieng, F.Y.F.; Latifah, S.Y.; Md Hashim, N.F.; Khaza’ai, H.; Ahmat, N.; Gopalsamy, B.; Wibowo, A. Ampelopsin E reduces the invasiveness of the triple negative breast cancer cell line, MDA-MB-231. Molecules 2019, 24, 2619. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, S.; Sorolla, A.; Fromont, J.; Blancafort, P.; Flematti, G.R. Aurantoside c targets and induces apoptosis in triple negative breast cancer cells. Mar. Drugs 2018, 16, 361. [Google Scholar] [CrossRef] [Green Version]
- Robles, A.J.; McCowen, S.; Cai, S.; Glassman, M.; Ruiz, F.; Cichewicz, R.H.; McHardy, S.F.; Mooberry, S.L. Structure-Activity Relationships of New Natural Product-Based Diaryloxazoles with Selective Activity against Androgen Receptor-Positive Breast Cancer Cells. J. Med. Chem. 2017, 60, 9275–9289. [Google Scholar] [CrossRef]
- Jang, H.; Ko, H.; Song, K.; Kim, Y.S. A sesquiterpenoid from farfarae flos induces apoptosis of MDA-MB-231 human breast cancer cells through inhibition of JAK-STAT3 signaling. Biomolecules 2019, 9, 278. [Google Scholar] [CrossRef] [Green Version]
- Fatima, I.; El-Ayachi, I.; Taotao, L.; Lillo, M.A.; Krutilina, R.; Seagroves, T.N.; Radaszkiewicz, T.W.; Hutnan, M.; Bryja, V.; Krum, S.A.; et al. The natural compound Jatrophone interferes with Wnt/β-catenin signaling and inhibits proliferation and EMT in human triple-negative breast cancer. PLoS ONE 2017, 12, e0189864. [Google Scholar] [CrossRef] [Green Version]
- Ling, T.; Hadi, V.; Guiguemde, A.; Landfear, S.M.; Rivas, F. Jatropha natural products as potential therapeutic leads. In The Formation, Structure and Activity of Phytochemicals. Recent Advances in Phytochemistry; Jetter, R., Ed.; Springer: Cham, Switzerland, 2015; Volume 45. [Google Scholar] [CrossRef]
- Li, H.; Yang, B.; Huang, J.; Xiang, T.; Yin, X.; Wan, J.; Luo, F.; Zhang, L.; Li, H.; Ren, G. Naringin inhibits growth potential of human triple-negative breast cancer cells by targeting β-catenin signaling pathway. Toxicol. Lett. 2013, 220, 219–228. [Google Scholar] [CrossRef]
- Jaspal, B.; Norman, F.; Kayla, A.; Maria, C.T.; Bela, P. A novel anti-triple negative breast cancer compound isolated from medicinal herb Myrothamnus flabellifolius. J. Med. Plants Res. 2018, 12, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Yu, W.; Cai, G.; Zhu, J.; Zhang, C.; Li, S.; Guo, J.; Yin, G.; Chen, C.; Kong, L. A new synthetic derivative of cryptotanshinone KYZ3 as STAT3 inhibitor for triple-negative breast cancer therapy. Cell Death Dis. 2018, 9, 1098. [Google Scholar] [CrossRef] [Green Version]
- Bonaccorsi, P.M.; Labbozzetta, M.; Barattucci, A.; Salerno, T.M.G.; Poma, P.; Notarbartolo, M. Synthesis of curcumin derivatives and analysis of their antitumor effects in triple negative breast cancer (TNBC) cell lines. Pharmaceuticals 2019, 12, 161. [Google Scholar] [CrossRef] [Green Version]
- Rios-Fuller, T.J.; Ortiz-Soto, G.; Lacourt-Ventura, M.; Maldonado-Martinez, G.; Cubano, L.A.; Schneider, R.J.; Martinez-Montemayor, M.M. Ganoderma lucidum extract (GLE) impairs breast cancer stem cells by targeting the STAT3 pathway. Oncotarget 2018, 9, 35907–35921. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Chen, Z.y.; Chen, L.; Zhang, J.y.; Fu, L.y.; Tao, L.; Zhang, Y.; Hu, X.x.; Shen, X.c. Shikonin inhibits triple-negative breast cancer-cell metastasis by reversing the epithelial-to-mesenchymal transition via glycogen synthase kinase 3β-regulated suppression of β-catenin signaling. Biochem. Pharmacol. 2019, 166, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wang, K.; Zhuang, J.; Gao, C.; Li, H.; Liu, L.; Feng, F.; Zhou, C.; Yao, K.; Deng, L.; et al. The modulatory properties of astragalus membranaceus treatment on triple-negative breast cancer: An integrated pharmacological method. Front. Pharmacol. 2019, 10, 1171. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Wang, C.Y.; Hu, R.; Lee, J.Y.; Luu, T.T.T.; Park, H.J.; Lee, S.K. Antitumor Activity of Vanicoside B Isolated from Persicaria dissitiflora by Targeting CDK8 in Triple-Negative Breast Cancer Cells. J. Nat. Prod. 2019, 82, 3140–3149. [Google Scholar] [CrossRef]
- Lou, C.; Chen, Y.; Zhang, J.; Yang, B.; Zhao, H. Eupalinolide J Suppresses the growth of triple-negative breast cancer cells via targeting STAT3 signaling pathway. Front. Pharmacol. 2019, 1071. [Google Scholar] [CrossRef]
- Li, H.C.; Xia, Z.H.; Chen, Y.F.; Yang, F.; Feng, W.; Cai, H.; Mei, Y.; Jiang, Y.M.; Xu, K.; Feng, D.X. Cantharidin Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing Autophagy and Inducing Apoptosis In Vitro and In Vivo. Cell. Physiol. Biochem. 2018, 43, 1829–1840. [Google Scholar] [CrossRef]
- Gangrade, A.; Pathak, V.; Augelli-Szafran, C.E.; Wei, H.X.; Oliver, P.; Suto, M.; Buchsbaum, D.J. Preferential inhibition of Wnt/β-catenin signaling by novel benzimidazole compounds in triple-negative breast cancer. Int. J. Mol. Sci. 2018, 19, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.; Chen, J.; Zhou, Z.; Xia, H.; Qiu, M.H.; Chen, C. Cucurbitacin e induces cell cycle G2/M phase arrest and apoptosis in triple negative breast cancer. PLoS ONE 2014, 9, e103760. [Google Scholar] [CrossRef]
- Huang, W.; Liang, Y.; Ma, X. Alpha-mangostin induces endoplasmic reticulum stress and autophagy which count against fatty acid synthase inhibition mediated apoptosis in human breast cancer cells. Cancer Cell Int. 2019, 19, 151. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H. Schisandrin A prevents oxidative stress-induced DNA damage and apoptosis by attenuating ROS generation in C2C12 cells. Biomed. Pharmacother. 2018, 106, 902–909. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.T.; Mulabagal, V.; Nalawade, S.M.; Chen, C.L.; Yang, T.F.; Tsay, H.S. Isolation and quantitative analysis of cryptotanshinone, an active quinoid diterpene formed in callus of Salvia miltiorrhiza BUNGE. Biol. Pharm. Bull. 2003, 26, 845–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auyeung, K.K.; Han, Q.-B.; Ko, J.K. Astragalus membranaceus: A review of its protection against inflammation and gastrointestinal cancers. Am. J. Chin. Med. 2016, 44, 1–22. [Google Scholar] [CrossRef]
- Wang, F.; Zhong, H.; Fang, S.; Zheng, Y.; Li, C.; Peng, G.; Shen, X. Potential anti-inflammatory sesquiterpene lactones from Eupatorium lindleyanum. Planta Med. 2018, 84, 123–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, S.; Chen, Y.; Yang, B.; Lou, C.; Zhu, R.; Zhao, Y.; Zhao, H. F1012-2 inhibits the growth of triple negative breast cancer through induction of cell cycle arrest, apoptosis, and autophagy. Phytother. Res. 2018, 32, 908–922. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Ji, L.; Luo, Y.; Hu, Y. Antioxidant activities of extracts and fractions from Eupatorium lindleyanum DC. Molecules 2011, 16, 5998–6009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.-P.; Li, L.; Xu, L.; Dai, E.N.; Chen, W.-D. Cantharidin suppresses cell growth and migration, and activates autophagy in human non-small cell lung cancer cells. Oncol. Lett. 2018, 15, 6527–6532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, E.E.; Mueller, L.E.; Treadwell, S.M.; Morris, C.A.; Machado, H.L. Molecular Targets of Triple-Negative Breast Cancer: Where Do We Stand? Cancers 2022, 14, 482. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, M.; Wang, B.; Zhang, L.; Fang, M.; Zhou, F. Chemoresistance and Metastasis in Breast Cancer Molecular Mechanisms and Novel Clinical Strategies. Front. Oncol. 2021, 11, 658552. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, X. Targeting the Wnt/β-catenin signaling pathway in cancer. J. Hematol. Oncol. 2020, 13, 165. [Google Scholar] [CrossRef]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wahba, H.A.; El-Hadaad, H.A. Current approaches in treatment of triple-negative breast cancer. Cancer Biol. Med. 2015, 12, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Maqbool, M.; Bekele, F.; Fekadu, G. Treatment Strategies Against Triple-Negative Breast Cancer: An Updated Review. Breast Cancer 2022, 14, 15–24. [Google Scholar] [CrossRef] [PubMed]




| TNBC Subtype | Cell Lines | Intrinsic Subtype | Expression of Gene | Potential Therapies |
|---|---|---|---|---|
| BL1 (Basal like-1) | HCC2157 HCC1599 HCC1937 HCC1143 HCC3153 MDA-MB-468 HCC38 HCC2185 | Basal A Basal A HER2 Basal A Basal A Basal A Unclassified/Basal B Basal A | Cell cycle DNA damage response (ATR-BRCA pathway) | PARP inhibitors, Platinum agents [42], Pan-HDAC inhibitor, Wnt/ β-Catenin inhibitor |
| BL2 (Basal like-2) | SUM149PT CAL851 HCC70 HCC1806 HDQ-P1 HCC1500 | Unclassified/Basal B Basal A Basal Unclassified/Basal A Unclassified Basal B | Growth factor Signaling pathways (EGFR, MET, NGF, Wnt/β-Catenin, IGF-IR) Glycolysis, Gluconeogenesis | PARP inhibitors, Platinum agents [42,43], mTOR inhibitors Growth-factor inhibitors [44], Wnt/ β-Catenin inhibitor |
| IM (Immunomodulatory) | HCC1187 DU4475 | Basal A Unclassified | Immune signaling (CTLA4, ILI 2, IL7 pathways antigen processing/presentation) cytokine signaling by JAK/STAT, TNF, and NF-κB pathways | (PD1/PD-L1 inhibitors, CTLA-4 inhibitor, LAG-3 inhibitor, Anti TIM-3 mAb, Hedgehog inhibitor) [14,42] |
| M (Mesenchymal like) | BT-549 CAL-51 CAL-120 | Unclassified/Basal B Unclassified Luminal B | EMT Growth factor signaling Cell motility Cell differentiation | Tyrosine kinase inhibitors PI3K/mTOR inhibitors EMT and CSC targeted MET inhibitor FGFR, EGFR, VEGFR inhibitor [14,41,42] |
| MSL (Mesenchymal Stem Cell-like) | Hs578T MDA-MB-157 SUM159PT MDA-MB-436 MDA-MB-231 | Unclassified/Basal B Unclassified/Basal B Unclassified/Basal B Unclassified/Basal B Unclassified/Basal B | EMT Growth factor Proliferation (decreased) Angiogenesis genes | Tyrosine kinase inhibitors PI3K/mTOR inhibitors Antiangiogenic Src antagonist MET inhibitor, Trop-2 inhibitor [14,41,45] |
| LAR (Luminal Androgen Receptor) | MDA-MB-453 HCC2185 CAL-14 SUM185PE MFM-223 | Luminal A Luminal A Luminal A Luminal A Luminal A/B | Androgen Receptor Luminal gene expression pattern Molecular apocrine subtype | Androgen Receptor targeted PI3K inhibitors [41,42] |
| Unclassified | HCC1395 BT20 SW527 | Basal HER2/Basal A Luminal B | - | - |
| Natural Product | Cell Lines | Mechanism | Methods | Reference |
|---|---|---|---|---|
| Ilamycin E (Streptomyces atratus) Actinomycetes | HCC1937 and MDA-MB-468 | Inhibition of endoplasmic reticulum (ER) stress and CHOP-BCl2 | In vitro | [202] |
| Schisandrin A | MDA-MB-231 | Inhibition of Wnt/ER stress | In vitro and in vivo (Xenograft mouse) | [203] |
| Ampelopsin E, Oligostilbene (Dryobalanops) | MDA-MB-231 | Inhibition of invadopodia formation by stopping migration, transmigration, and invasive expressions of PDGF MMP2, MMP9, MMP14 | In vitro | [204] |
| Aurantoside C (C828) (Sponge Manihinealynbeazleyae) | MDA-MB-231, SUM159PT and SUM149 | Inhibition of the phosphorylation of Akt/mTORdan NF-κB pathways and increased the phosphorylation of p38 MAPK and SAPK/JNK pathway | In vitro | [205] |
| Amyris texana (Oxazole) Discovery of Compound 30 (CIDD-0067106) | MDA-MB-453 | Inhibition of the activity of the mTORC1 pathway, a model of the Luminal Androgen Receptor (LAR) | In vitro and in silico | [206] |
| A sequesterpenoid from Farfarae Flos (Tussilago farfara) | MDA-MB-231 | Inhibition of JAK-STAT3 signaling | In vitro and in vivo (Tumor Xenograft) | [207] |
| Diterpen Jatrophone (Jatropha isabelli) | MDA-MB-231, HCC38, MDA-MB-157 and MDA-MB-468 | Inhibition of Wnt/β-Catenin signaling and proliferation and EMT | In vitro | [208,209] |
| Naringin/Flavonoid (Dynaria fortunei, citrus aurantium, citrus medica L.) | MDA-MB-231 | Inhibition of growth potential by targeting β-Catenin signaling pathway | In vitro and in vivo (Xenograft mice) | [210] |
| Myrothamnus flabelli folius (Derivative of Galloyl glucose hexahydroxydiphenic acid) | BT-549T and MDA-MB-231 | Inhibited the growth cell | In vitro | [211] |
| Cryptotanshinone (Salviamiltiorrhiza Bunge) | MDA-MB-231 | Inhibition KYZ3 by decreasing the level of MMP-9 with activated STAT3 | In vitro, in silico, and in vivo (Subcutaneous implantation), | [212] |
| Curcuma longa | SUM149 and MDA-MB-231 | Inhibition of NF-κB transcriptional factor activity and consequently the expression of some NF-κB targets | In vitro | [213] |
| Ganoderma lucidum | SUM149 and MDA-MB-231 | Inhibition of STAT3 and JAK2 | In vitro and in vivo (Injected limiting dilutions combined immunodeficient (CD44+/CD24–) | [214] |
| Annonamuricata leaf | MDA-MB-231 | Intrinsic Apoptotic pathway | In vitro | [158] |
| Shikonin (Lithospermum erythrorhizon Sieb. et Zucc) | MDA-MB-231 and 4T1 | Inhibition of the epithelial-to-mesenchymal transition via glycogen synthase kinase 3β-regulated suppression of β-catenin signaling | In vitro | [215] |
| Astragalus membranaceus | MDA-MB-231 | Inhibition of PIK3CG/AKT/BCL2 signaling pathway | In vitro and in silico | [216] |
| Vanicoside B (Persicaria dissitiflora) | MDA-MB-231 and HCC38 | Inhibition CDK8-signaling pathway | In vitro and in vivo (Tumor Xenograft Model) | [217] |
| Eupalinolide J (Eupatorium lindleyanum DC) | MDA-MB-231 and MDA-MB-468 | Suppressing growth by STAT3 signaling pathways such as anti-apoptosis, cell cycle arrest, and MMP disruption | In vitro and in vivo (Xenograft Mouse Model) | [218] |
| Cantharidin Component of terpenoidsecreted by the blister beetle Mylabris phalerata (Pallas) | MDA-MB-231 and MDA-MB-468 | Suppressing Autophagy and Inducing apoptosis by inhibiting the conversion of LC3 I to LC3 II and suppressing the expression of Beclin-1 | In vitro and in vivo (Subcutaneous inoculation) | [219] |
| Benzimidazole compounds (SRI33576 and SRI35889) | MDA-MB-231 and MDA-MB-468 | Inhibition of Wnt/β-Catenin signaling and also detract of mTOR, STAT3 and Notch signaling | In vitro | [220] |
| Cucurbitacin E from Hemsleya delavayi var. yalungensis (Cucurbitaceae) | MDA-MB-468 and SW527 | Induced cell cycle G2/M phase arrest and apoptosis by expression of Cyclin D1, Survivin, XIAP, Bcl2, and Mcl-1 and increased activation of JNK and inhibited activation of AKT and ERK within MDA-MB-468 | In vitro | [221] |
| α-mangostin (Garcinia mangostana L.) | MDA-MB-231 and MCF-7 | Induced endoplasmic reticulum stress and autophagy by fatty acid synthase inhibition mediated apoptosis | In vitro | [222] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dewi, C.; Fristiohady, A.; Amalia, R.; Khairul Ikram, N.K.; Ibrahim, S.; Muchtaridi, M. Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line. Molecules 2022, 27, 3661. https://doi.org/10.3390/molecules27123661
Dewi C, Fristiohady A, Amalia R, Khairul Ikram NK, Ibrahim S, Muchtaridi M. Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line. Molecules. 2022; 27(12):3661. https://doi.org/10.3390/molecules27123661
Chicago/Turabian StyleDewi, Citra, Adryan Fristiohady, Riezki Amalia, Nur Kusaira Khairul Ikram, Sugeng Ibrahim, and Muchtaridi Muchtaridi. 2022. "Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line" Molecules 27, no. 12: 3661. https://doi.org/10.3390/molecules27123661
APA StyleDewi, C., Fristiohady, A., Amalia, R., Khairul Ikram, N. K., Ibrahim, S., & Muchtaridi, M. (2022). Signaling Pathways and Natural Compounds in Triple-Negative Breast Cancer Cell Line. Molecules, 27(12), 3661. https://doi.org/10.3390/molecules27123661