Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design
Abstract
:1. Introduction
2. Results
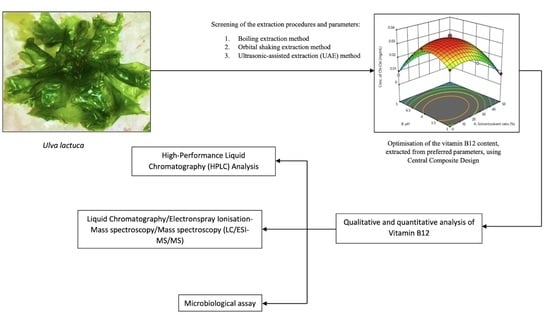
2.1. Screening the Extraction Methods and Drying Conditions
2.2. Screening for Significant Factors Using the Two-Level Factorial Design
2.3. Significant Factors That Affected the CN-Cbl Yield from the Oven-Dried U. lactuca Samples That Employed the UAE Technique
2.4. Optimising of the ODU CN-Cbl Yield through CCD
2.5. Analysis of the Optimum Region by Employing CCD
2.6. Model Conformation and Validation Test
2.7. Microbiological Assay of Vitamin B12
2.8. Liquid Chromatography/Electronspray Ionisation-Mass Spectroscopy/Mass Spectroscopy (LC/ESI-MS/MS) Analysis of Vitamin B12
3. Discussion
4. Materials and Methods
4.1. Sample Collection and Preparation
- Air drying: The U. lactuca samples were placed in a single layer on a clean flipchart paper and air-dried in the laboratory using fan (speed 300 rpm) to keep air circulating until the sample reached a constant weight (temperature 25 °C).
- Sun-drying: The U. lactuca samples were placed in a single layer on a clean aluminium foil and dried under direct sunlight until the sample reached a constant weight (temperature 32 ± 2 °C).
- Oven drying: The U. lactuca samples were placed on an aluminium foil in a single layer and dried in an oven drier (Sanyo, OSA, JP) until a constant weight was obtained. The oven temperature was set to 40 °C.
- Freeze drying: The U. lactuca samples were frozen in a freezer (Sanyo, OSA, JP) overnight at −80 °C and then being placed in a chamber that combines a chilled condenser and a vacuum pump to aid sublimation of water. The freeze-drier (Thermo Scientific, WLM, USA) was set at a cryo-temperature of −50 °C.
4.2. Extraction of Vitamin B12
- Boiling Extraction Method: The dried U. lactuca samples were boiled using hotplate (Thermolyne Thermo Fischer Scientific, Waltham, MA, USA) for 20 min at 65 °C [14]. Upon extraction, the extracts obtained were centrifuged and kept in below 15 °C chiller (Sanyo, OSA, JP) before being purified.
- Orbital Shaker Extraction Method: The dried U. lactuca samples were shaken on an orbital shaker (Thermo Fischer Scientific, WLM, USA) for 30 min [28] at 200 rpm and centrifuged upon extraction. The extracts were kept in below 15 °C chiller before being purified.
- Ultrasonic Assisted Extraction Method: The dried U. lactuca samples were suspended in the solvent mixture and extracted in an ultrasonic water bath (WiseClean, CH) with the power and frequency set to 665 Watt and 20 kHz, respectively, for 30 min. The extracts obtained were cooled and kept in a chiller below 15 °C before being purified.
4.3. Purification and Determination of Vitamin B12
4.4. High-Performance Liquid Chromatography (HPLC) Analysis
4.4.1. Sample Solutions
4.4.2. Standard Solutions
4.4.3. Chromatographic Conditions
4.5. Statistical Analysis
4.6. Microbiological Assays of Vitamin B12 Using Eschericia coli and Lactobacillus leichmanii
4.7. Tandem Mass Spectrometry/Mass Spectroscopy (MS/MS) Analysis of Vitamin B12
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
List of Abbreviations
| ODB | Oven-dried: extracted by boiling method |
| SDB | Sun-dried sample, extracted by boiling method |
| ADB | Air-dried sample, extracted by boiling method |
| FDB | Freeze-dried, extracted by boiling method |
| ODO | Oven-dried, extracted by orbital shaking method |
| SDO | Sun-dried, extracted by orbital shaking method |
| ADO | Air-dried, extracted by orbital shaking method |
| FDO | Freeze-dried, extracted by orbital shaking method |
| ODU | Oven-dried, extracted by ultrasonic-assisted extraction method |
| SDU | Sun-dried, extracted by ultrasonic-assisted extraction method |
| ADU | Air-dried, extracted by ultrasonic-assisted extraction method |
| FDU | Freeze-dried, extracted by ultrasonic-assisted extraction method |
References
- Shahar, S.; Budin, S.B.; Bakar, M.A.; Umar, N.A.; Halim, J.M. Anaemia and Cognitive Function among Chinese Elderly in Old Folks Homes. J. Sains Kesihat. Malaysia Malays. J. Health Sci. 2005, 3, 1–15. [Google Scholar]
- Del Bo, C.; Riso, P.; Gardana, C.; Brusamolino, A.; Battezzati, A.; Ciappellano, S. Effect of Two Different Sublingual Dosages of Vitamin B12 on Cobalamin Nutritional Status in Vegans and Vegetarians with a Marginal Deficiency: A Randomized. Clin. Nutr. 2019, 38, 575–583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, C.W. Vitamin B12 Deficiency in the Elderly: Is It Worth Screening. Hong Kong Med. J. 2015, 21, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Langan, R.C.; Goodbred, A.J. Vitamin B12 Deficiency: Recognition and Management. Am. Fam. Physician 2017, 96, 384–389. [Google Scholar] [CrossRef] [PubMed]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 5311498, Cyanocobalamin. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Cyanocobalamin (accessed on 11 July 2022).
- Jayasinghe, P.S.; Pahalawattaarachchi, V.; Ranaweera, K.K.D.S. Seaweed Based Jam as a Source of Nutrition. Available online: https://scholar.google.com/scholar?cluster=6002363050367060791&hl=en&as_sdt=0,5 (accessed on 10 May 2022).
- Ganesan, A.R.; Tiwari, U.; Rajauria, G. Seaweed Nutraceuticals and Their Therapeutic Role in Disease Prevention. Food Sci. Hum. Wellness 2019, 8, 252–263. [Google Scholar] [CrossRef]
- Wahlström, N.; Nylander, F.; Malmhäll-Bah, E.; Sjövold, K.; Edlund, U.; Westman, G.; Albers, E. Composition and Structure of Cell Wall Ulvans Recovered from Ulva Spp. along the Swedish West Coast. Carbohydr. Polym. 2020, 233, 115852. [Google Scholar] [CrossRef]
- Yu-Qing, T.; Mahmood, K.; Shehzadi, R.; Ashraf, M.F. Ulva Lactuca and Its Polysaccharides: Food and Biomedical Aspects. J. Biol. 2016, 6, 140–151. [Google Scholar]
- Macartain, P.; Gill, C.I.R.; Brooks, M.; Campbell, R.; Rowland, I.R. Nutritional Value of Edible Seaweeds. Nutr. Rev. 2007, 65, 535–543. [Google Scholar] [CrossRef]
- Golshani, T.; Jorjani, E.; Chelgani, S.C.; Shafaei, S.Z.; Nafechi, Y.H. Modeling and Process Optimization for Microbial Desulfurization of Coal by Using a Two-Level Full Factorial Design. Int. J. Min. Sci. Technol. 2013, 23, 261–265. [Google Scholar] [CrossRef]
- Gottipati, R.; Mishra, S. Process Optimization of Adsorption of Cr (VI) on Activated Carbons Prepared from Plant Precursors by a Two-Level Full Factorial Design. Chem. Eng. J. 2020, 160, 99–107. [Google Scholar] [CrossRef]
- Jalilian, N.; Najafpour, G.D.; Khajouei, M. Enhanced Vitamin B12 Production Using Chlorella Vulgaris. Int. J. Eng. Trans. A Basics 2019, 32, 1–9. [Google Scholar] [CrossRef]
- Kumudha, A.; Sarada, R. Effect of Different Extraction Methods on Vitamin B12 from Blue Green Algae, Spirulina Platensis. Pharm. Anal. Acta 2015, 6, 337. [Google Scholar] [CrossRef] [Green Version]
- Chandra-Hioe, M.V.; Xu, H.; Arcot, J. The Efficiency of Ultrasonic-Assisted Extraction of Cyanocobalamin Is Greater than Heat Extraction. Heliyon 2020, 6, e03059. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dang, T.T.; Van Vuong, Q.; Schreider, M.J.; Bowyer, M.C.; Van Altena, I.A.; Scarlett, C.J. Optimisation of Ultrasound-Assisted Extraction Conditions for Phenolic Content and Antioxidant Activities of the Alga Hormosira Banksii Using Response Surface Methodology. J. Appl. Phycol. 2017, 29, 3161–3173. [Google Scholar] [CrossRef]
- Madhubalaji, C.K.; Mudaliar, S.N.; Chauhan, V.S.; Sarada, R. Evaluation of Drying Methods on Nutritional Constituents and Antioxidant Activities of Chlorella Vulgaris Cultivated in an Outdoor Open Raceway Pond. J. Appl. Phycol. 2021, 33, 1419–1434. [Google Scholar] [CrossRef]
- Azwanida, N. A Review on the Extraction Methods Use in Medicinal Plants, Principle, Strength and Limitation. Med. Aromat. Plants 2015, 4, 3–8. [Google Scholar] [CrossRef]
- Watanabe, F.; Yabuta, Y.; Bito, T.; Teng, F. Vitamin B12-Containing Plant Food Sources for Vegetarians. Nutrients 2014, 6, 1861–1873. [Google Scholar] [CrossRef] [Green Version]
- Ruslan, F.S.; Susanti, D.; Taher, M.; Mohammad, N.F. Optimization of Supercritical Fluid Extraction of Asiaticoside from Centella Asiatica Using Central Composite Design (CCD). Sep. Sci. Technol. 2021, 56, 2766–2774. [Google Scholar] [CrossRef]
- Rodrigues, D.; Sousa, S.; Silva, A.; Amorim, M.; Pereira, L.; Rocha-Santos, T.A.P.; Gomes, A.M.P.; Duarte, A.C.; Freitas, A.C. Impact of Enzyme- and Ultrasound-Assisted Extraction Methods on Biological Properties of Red, Brown, and Green Seaweeds from the Central West Coast of Portugal. J. Agric. Food Chem. 2015, 63, 3177–3188. [Google Scholar] [CrossRef]
- Ciko, A.M.; Jokić, S.; Šubarić, D.; Jerković, I. Overview on the Application of Modern Methods for the Extraction of Bioactive Compounds from Marine Macroalgae. Mar. Drugs 2018, 16, 348. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.F.R.; Abreu, H.; Silva, A.M.S.; Cardoso, S.M. Effect of Oven-Drying on the Recovery of Valuable Compounds from Ulva Rigida, Gracilaria Sp. and Fucus Vesiculosus. Mar. Drugs 2019, 17, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, F.; Kang, X.J.; Liu, Z.Y.; Ma, Y.Q.; Gu, Z.Z. Use of Packed-Fiber Solid-Phase Extraction for Sample Clean-up and Preconcentration of Vitamin B12 before Determination. Chinese Chem. Lett. 2009, 20, 1491–1494. [Google Scholar] [CrossRef]
- Lovander, M.D.; Lyon, J.D.; Parr, D.L.; Wang, J.; Parke, B.; Leddy, J. Critical Review—Electrochemical Properties of 13 Vitamins: A Critical Review and Assessment. J. Electrochem. Soc. 2018, 165, G18–G49. [Google Scholar] [CrossRef]
- Bajaj, S.R.; Singhal, R.S. Degradation Kinetics of Vitamin B12 in Model Systems of Different PH and Extrapolation to Carrot and Lime Juices. J. Food Eng. 2020, 272, 109800. [Google Scholar] [CrossRef]
- House, E.; Estate, W.I.; Jigani, B.; Rd, L.; Technologies, A.; Park, B.; Rd, J.L. No Title. Single-Laboratory Valid. AOAC. 2020, 1, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Ohmori, S.; Kataoka, M.; Koyama, H. Stability of Cyanocobalamin in Sugar-Coated Tablets. Int. J. Pharm. 2007, 337, 161–168. [Google Scholar] [CrossRef]
- Watanabe, F.; Schwarz, J.; Takenaka, S.; Miyamoto, E.; Ohishi, N.; Nelle, E.; Hochstrasser, R.; Yabuta, Y. Characterization of Vitamin B12 Compounds in the Wild Edible Mushrooms Black Trumpet (Craterellus Cornucopioides) and Golden Chanterelle (Cantharellus Cibarius). J. Nutr. Sci. Vitaminol. 2012, 58, 438–441. [Google Scholar] [CrossRef] [Green Version]







| Run | Solvent:Solvent Ratio (MeOH:H2O) | pH | Conc. of Vitamin B12 (mg/mL) |
|---|---|---|---|
| 1 | 0:100 | 3 | 0.0250 |
| 2 | 50:50 | 3 | 0.0084 |
| 3 | 0:100 | 5 | 0.0072 |
| 4 | 50:50 | 5 | 0.0055 |
| 5 | 0:100 | 4 | 0.0323 |
| 6 | 50:50 | 4 | 0.0189 |
| 7 | 25:75 | 3 | 0.0225 |
| 8 | 25:75 | 5 | 0.0192 |
| 9 | 25:75 | 4 | 0.0357 |
| 10 | 25:75 | 4 | 0.0355 |
| 11 | 25:75 | 4 | 0.0351 |
| 12 | 25:75 | 4 | 0.0358 |
| 13 | 25:75 | 4 | 0.0305 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | |
|---|---|---|---|---|---|---|
| Model | 0.0003 | 5 | 0.0003 | 49.26 | <0.0001 | Significant |
| A | 0.0002 | 1 | 0.0002 | 27.14 | 0.0012 | |
| B | 0.0001 | 1 | 0.0001 | 15.64 | 0.0055 | |
| AB | 0.0001 | 1 | 0.0001 | 9.06 | 0.0196 | |
| A² | 0.0002 | 1 | 0.0002 | 37.27 | 0.0005 | |
| B² | 0.0005 | 1 | 0.0005 | 85.86 | <0.0001 | |
| Residual | 0.0000 | 7 | 6.179 × 10−7 | |||
| Lack of fit | 0.0000 | 3 | 7.511 × 10−6 | 1.45 | 0.3538 | Not significant |
| Pure error | 0.0000 | 4 | 5.180 × 10−6 | |||
| Cor total | 0.0016 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Susanti, D.; Ruslan, F.S.; Shukor, M.I.; Nor, N.M.; Aminudin, N.I.; Taher, M.; Khotib, J. Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design. Molecules 2022, 27, 4459. https://doi.org/10.3390/molecules27144459
Susanti D, Ruslan FS, Shukor MI, Nor NM, Aminudin NI, Taher M, Khotib J. Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design. Molecules. 2022; 27(14):4459. https://doi.org/10.3390/molecules27144459
Chicago/Turabian StyleSusanti, Deny, Fatin Shazwani Ruslan, Muhammad Idham Shukor, Normawaty Mohammad Nor, Nurul Iman Aminudin, Muhamad Taher, and Junaidi Khotib. 2022. "Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design" Molecules 27, no. 14: 4459. https://doi.org/10.3390/molecules27144459
APA StyleSusanti, D., Ruslan, F. S., Shukor, M. I., Nor, N. M., Aminudin, N. I., Taher, M., & Khotib, J. (2022). Optimisation of Vitamin B12 Extraction from Green Edible Seaweed (Ulva lactuca) by Applying the Central Composite Design. Molecules, 27(14), 4459. https://doi.org/10.3390/molecules27144459