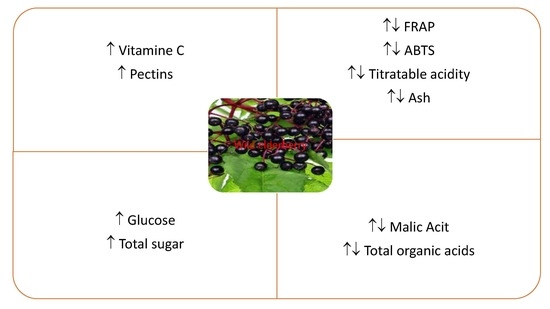
Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry
Abstract
:1. Introduction
2. Results and Discussion
2.1. Basic Determinations and Antioxidant Activity
2.2. Sugars and Organic Acid
2.3. Triterpenes
2.4. Carotenoids
3. Materials and Methods
3.1. Reagents and Standard
3.2. Plant Materials
3.3. Dry-Matter, Ash Content, Titratable Acidity, Pectin and Vitamin C
3.4. Antioxidant-Activity Analysis
3.5. Analysis of Sugars with HPLC-ELSD Method
3.6. Analysis of Organic Acids by HPLC Method
3.7. Analysis of Triterpenoids by the UPLC-PDA-MS/MS Method
3.8. Carotenoid Content UPLC−PDA−MS Analysis
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Charlebois, D.; Byers, P.L.; Finn, C.E.; Thomas, A.L. Elderberry: Botany, Horticulture, Potential. Hortic. Rev. 2010, 37, 213–280. [Google Scholar] [CrossRef]
- Topolska, J.; Kostecka-Gugała, A.; Ostachowicz, B.; Latowski, D. Selected metal content and antioxidant capacity of Sambucus nigra flowers from the urban areas versus soil parameters and traffic intensity. Environ. Sci. Pollut. Res. 2020, 27, 668–677. [Google Scholar] [CrossRef] [PubMed]
- Maslennikov, P.V.; Chupakhina, G.N.; Skrypnik, L.N.; Feduraev, P.V.; Melnik, A.S. Assessment of the Antioxidant Potential of Plants in Urban Ecosystems under Conditions of Anthropogenic Pollution of Soils. Russ. J. Ecol. 2018, 49, 384–394. [Google Scholar] [CrossRef]
- Olejnik, A.; Olkowicz, M.; Kowalska, K.; Rychlik, J.; Dembczyński, R.; Myszka, K.; Juzwa, W.; Białas, W.; Moyer, M.P. Gastrointestinal digested Sambucus nigra L. fruit extract protects in vitro cultured human colon cells against oxidative stress. Food Chem. 2016, 197, 648–657. [Google Scholar] [CrossRef]
- Ercisli, S.; Tosun, M.; Akbulut, M. Physico-chemical characteristics of some wild grown European elderberry (Sambucus nigra L.) genotypes. Pharmacogn. Mag. 2009, 5, 320–337. [Google Scholar] [CrossRef]
- Veberic, R.; Jakopic, J.; Stampar, F.; Schmitzer, V. European elderberry (Sambucus nigra L.) rich in sugars, organic acids, anthocyanins and selected polyphenols. Food Chem. 2009, 114, 511–515. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Schmitzer, V.; Slatnar, A.; Todorovic, B.; Veberic, R.; Stampar, F.; Ivancic, A. Investigation of Anthocyanin Profile of Four Elderberry Species and Interspecific Hybrids. J. Agric. Food Chem. 2014, 62, 5573–5580. [Google Scholar] [CrossRef] [PubMed]
- Salvador, C.; Rocha, S.M.; Silvestre, A.J.D. Lipophilic phytochemicals from elderberries (Sambucus nigra L.): Influence of ripening, cultivar and season. Ind. Crop. Prod. 2015, 71, 15–23. [Google Scholar] [CrossRef]
- Kołodziej, B.; Drożdżal, K. Właściwości przeciwutleniające kwiatów i owoców bzu czarnego pozyskiwanego ze stanu naturalnego. Żywność Nauka Technol. Jakość 2011, 4, 36–44. [Google Scholar]
- Nile, S.H.; Park, S.W. Edible berries: Bioactive components and their effect on human health. Nutrition 2014, 30, 134–144. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture, Agricultural Research Service (USDA). USDA National Nutrient Database for Standard Reference, Release 23. Nutrient Data Laboratory Home Page. 2010. Available online: http://www.ars.usda.gov/ba/bhnrc/ndl (accessed on 25 February 2022).
- Krüger, S.; Mirgos, M.; Morlock, G.E. Effect-directed analysis of fresh and dried elderberry (Sambucus nigra L.) via hyphenated planar chromatography. J. Chromatogr. A 2015, 1426, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Netzel, M.; Strass, G.; Herbst, M.; Dietrich, H.; Bitsch, R.; Bitsch, I.; Frank, T. The excretion and biological antioxidant activity of elderberry antioxidants in healthy humans. Food Res. Int. 2005, 38, 905–910. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Łysiak, G. Bioactive properties of Sambucus nigra L. as a functional ingredient for food and pharmaceutical industry. J. Funct. Foods 2018, 40, 377–390. [Google Scholar] [CrossRef] [PubMed]
- Vulić, J.; Vračar, L.; Šumić, Z. Chemical characterictics of cultivated elderberry fruit. Acta Period. Technol. 2008, 39, 85–90. [Google Scholar] [CrossRef]
- Diviš, P.; Vespalcová, M.; Pořízka, J.; Matějíček, A.; Kaplan, J. Elemental composition of fruits from different Black elder (Sambucus nigra L.) cultivars grown in the Czech Republic. J. Elem. 2015, 20, 549–557. [Google Scholar] [CrossRef]
- Gleńsk, M.; Czapińska, E.; Woźniak, M.; Ceremuga, I.; Włodarczyk, M.; Terlecki, G.; Ziółkowski, P.; Seweryn, E. Triterpenoid Acids as Important Antiproliferative Constituents of European Elderberry Fruits. Nutr. Cancer 2017, 69, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Lachowicz, S.; Oszmiański, J.; Seliga, Ł.; Pluta, S. Phytochemical Composition and Antioxidant Capacity of Seven Saskatoon Berry (Amelanchier alnifolia Nutt.) Genotypes Grown in Poland. Molecules 2017, 22, 853. [Google Scholar] [CrossRef] [Green Version]
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2005. [Google Scholar]
- PN-90/A-75101/04; Fruit and Vegetable Products—Preparation of Samples and Test Methods—Determination of Total Acidity. Polski Komitet Normalizacyjny: Warsaw, Poland, 1990.
- Pijanowski, E.; Mrożewski, S.; Horubała, A.; Jarczyk, A. Technologia Produktów Owocowych i Warzywnych; T. I. PWRiL: Warszawa, Poland, 1973. [Google Scholar]
- PN-90/A-75101/11; Fruit and Vegetable Products—Preparation of Samples and Testing Methods—Determination of Ascorbic Acid Content. Polski Komitet Normalizacyjny: Warsaw, Poland, 1990.
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Chemical composition and antioxidant capacity of different anatomical parts of pear (Pyrus communis L.). Food Chem. 2016, 203, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Nawirska-Olszańska, A.; Kolniak-Ostek, J.; Oziembłowski, M.; Tichá, A.; Hyšpler, R.; Zadak, Z.; Židová, P.; Paprstein, F. Comparison of old cherry cultivars grown in Czech Republic by chemical composition and bioactive compounds. Food Chem. 2017, 228, 136–142. [Google Scholar] [CrossRef] [PubMed]


| Variety | Dry Matter | Ash | Titratable Acidity | Pectins | Vitamin C | FRAP | ABTS |
|---|---|---|---|---|---|---|---|
| % | % | g/100 g FW * | % | mg/100 g FW | µMol/100 g FW | µMol/100 g FW | |
| Albida | 17.71 ± 1.98 f | 1.25 ± 0.23 a | 0.52 ± 0.08 d | 0.83 ± 0.09 d,e | 13.86 ± 2.14 c | 1373.96 ± 48.09 e | 707.26 ± 5.40 e |
| Bohatka | 18.88 ± 2.47 e | 0.86 ± 0.09 c | 0.96 ± 0.09 a | 0.88 ± 0.08 d | 14.42 ± 1.68 c | 4396.74 ± 201.9 c | 2097.12 ± 15.63 c |
| Haschberg | 22.32 ± 3.78 a | 1.28 ± 0.16 a | 0.93 ± 0.10 a | 1.00 ± 0.09 b | 15.70 ± 2.98 c | 6426.89 ± 108.2 b | 2849.66 ± 26.14 b |
| Sambo | 20.72 ± 1.76 c | 0.88 ± 0.09 c | 0.94 ± 0.09 a | 0.94 ± 0.09 c | 23.28 ± 3.76 b | 7276.70 ± 75.79 a | 3359.04 ± 37.41 a |
| Samdal | 20.04 ± 1.95 d | 1.02 ± 0.09 b | 0.82 ± 0.09 c | 0.34 ± 0.08 f | 10.19 ± 1.72 d | 1819.41 ± 89.67 e | 746.32 ± 3.25 e |
| Weihenstephan | 21.14 ± 2,98 b | 1.29 ± 0.09 a | 0.87 ± 0.09 b | 0.86 ± 0.09 d | 8.56 ± 1.04 e | 3669.43 ± 100.0 d | 1684.81 ± 17.17 d |
| Wildly growing P | 21.16 ± 4.25 b | 0.93 ± 0.08 c | 0.84 ± 0.09 c | 1.54 ± 1.12 a | 44.26 ± 5.98 a | 3731.10 ± 99.95 d | 1529.09 ± 11.92 d |
| Wildly growing C | 21.13 ± 2.58 b | 0.89 ± 0.07 c | 0.82 ± 0.09 c | 1.34 ± 1.06 a | 39.67 ± 2.66 a | 3685.19 ± 67.93 d | 1678.04 ± 13.79 d |
| Variety | Fructose | Glucose | Sorbitol | Sum Sugars | Citric Acid | Malic Acid | Shikimic Acid | Fumaric Acid | Sum Organic Acid |
|---|---|---|---|---|---|---|---|---|---|
| g/100 g FW | mg/100 g FW | ||||||||
| Albida | 2.84 ± 0.16 a | 1.31 ± 0.78 c | 0.40 ± 0.09 a | 4.55 ± 0.47 b | 265.65 ± 19.56 e | 99.56 ± 8.76 d | 1.67 ± 0.12 c | 0.87 ± 0.06 c,d | 367.75 ± 32.85 c |
| Bohatka | 0.99 ± 0.07 f | 0.11 ± 0.09 e | 0.00 | 1.11 ± 0.12 d | 502.72 ± 46.32 a | 141.87 ± 13.67 a | 10.01 ± 0.97 a | 4.34 ± 0.32 a | 658.94 ± 62.84 a |
| Haschberg | 1.93 ± 0.19 d | 1.85 ± 0.08 b | 0.00 | 3.79 ± 0.29 b | 437.48 ± 29.63 b | 139.85 ± 13.34 a | 10.25 ± 1.09 a | 3.72 ± 0.29 b | 591.30 ± 45.32 a |
| Sambo | 1.71 ± 0.12 c | 2.02 ± 0.07 b | 0.00 | 3.73 ± 0.26 b | 466.23 ± 34.97 b | 138.77 ± 13.21 a | 9.45 ± 0.93 a | 4.01 ± 0.30 a | 618.46 ± 54.76 a |
| Samdal | 1.92 ± 0.14 d | 1.88 ± 0.09 b | 0.00 | 3.79 ± 0.31 b | 324.98 ± 30.74 d | 105.12 ± 9.99 d | 5.32 ± 0.51 b | 1.06 ± 0.09 c | 436.48 ± 38.65 c |
| Weihenstephan | 1.23 ± 0.16 e | 1.09 ± 0.09 d | 0.00 | 2.32 ± 0.19 c | 392.11 ± 28.81 c | 122.18 ± 12.09 b | 4.36 ± 0.39 b | 1.02 ± 0.09 c | 519.67 ± 49.12 b |
| Wildly growing P | 2.42 ± 0.36 b | 3.45 ± 0.19 a | 0.00 | 5.87 ± 0.49 a | 365.36 ± 29.63 d | 113.63 ± 11.07 c | 1.99 ± 0.13 c | 0.95 ± 0.05 c | 481.93 ± 39.67 c |
| Wildly growing C | 2.25 ± 0.12 b | 3.01 ± 0.08 a | 0.00 | 5.26 ± 0.35 a | 336.36 ± 26.23 d | 111.99 ± 10.12 c | 1.77 ± 0.09 c | 0.90 ± 0.08 c,d | 451.02 ± 35.02 c |
| Variety | Betulinic Acid | Oleanolic Acid | Ursolic Acid | Sum |
|---|---|---|---|---|
| µg/g FW | ||||
| Albida | 49.27 ± 6.04 c | 433.72 ± 52.71 a | 1091.14 ± 110.6 a | 1574.13 ± 123.2 a |
| Bohatka | 44.15 ± 3.43 d | 305.38 ± 32.96 b | 925.71 ± 100.1 b | 1275.24 ± 112.6 b |
| Haschberg | 54.35 ± 4.27 b | 172.62 ± 19.33 d | 601.69 ± 59.94 d | 828.66 ± 40.68 d |
| Sambo | 50.14 ± 3.99 c | 192.10 ± 20.73 d | 579.28 ± 52.52 d | 821.52 ± 76.34 d |
| Samdal | 29.97 ± 2.51 e | 137.61 ± 12.97 e | 370.70 ± 32.87 e | 538.28 ± 51.42 e |
| Weihenstephan | 56.44 ± 6.01 b | 237.93 ± 21.66 c | 765.55 ± 61.63 c | 1059.92 ± 100.9 c |
| Wildly growing P | 68.65 ± 5.44 a | 246.32 ± 19.83 c | 734.64 ± 74.92 c | 1049.61 ± 99.61 c |
| Wildly growing C | 66.45 ± 5.27 a | 239.49 ± 18.36 c | 753.82 ± 24.88 c | 1059.76 ± 102.6 c |
| Variety | All-Trans-Lutein | β-Cryptoxantine | α-Carotene | 15-Cis-β-Carotene | Sum |
|---|---|---|---|---|---|
| mg/kg FW | |||||
| Albida | 4.67 ± 0.38 b,c | 47.36 ± 4.44 f | 0.00 | 0.00 | 52.03 ± 4.98 e |
| Bohatka | 10.55 ± 1.05 b | 280.66 ± 26.51 c,d | 8.09 ± 0.87 b | 29.55 ± 2.42 a | 328.38 ± 51.9 c |
| Haschberg | 7.61 ± 0.79 b | 171.80 ± 15.51 d | 1.92 ± 0.09 e | 2.44 ± 0.12 d | 183.77 ± 16.9 d |
| Sambo | 9.56 ± 1.01 b | 303.91 ± 29.76 c | 6.29 ± 0.56 c | 8.65 ± 0.82 b | 328.42 ± 31.87 c |
| Samdal | 14.84 ± 1.34 b | 414.19 ± 39.72 b | 4.43 ± 0.33 d | 5.54 ± 0.53 c | 439.00 ± 42.7 b |
| Weihenstephan | 5.42 ± 0.46 b | 127.63 ± 12.07 d,e | 3.18 ± 0.29 d | 1.55 ± 0.09 d | 137.77 ± 12.8 d |
| Wildly growing P | 31.50 ± 2.98 a | 841.61 ± 7.45 a | 14.08 ± 1.03 a | 8.41 ± 0.74 b | 895.60 ± 76.3 a |
| Wildly growing C | 28.88 ± 2.53 a | 789.58 ± 6.89 a | 12.48 ± 1.19 a | 8.58 ± 0.98 b | 839.51 ± 79.9 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nawirska-Olszańska, A.; Oziembłowski, M.; Brandova, P.; Czaplicka, M. Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry. Molecules 2022, 27, 5050. https://doi.org/10.3390/molecules27165050
Nawirska-Olszańska A, Oziembłowski M, Brandova P, Czaplicka M. Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry. Molecules. 2022; 27(16):5050. https://doi.org/10.3390/molecules27165050
Chicago/Turabian StyleNawirska-Olszańska, Agnieszka, Maciej Oziembłowski, Pavla Brandova, and Marta Czaplicka. 2022. "Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry" Molecules 27, no. 16: 5050. https://doi.org/10.3390/molecules27165050
APA StyleNawirska-Olszańska, A., Oziembłowski, M., Brandova, P., & Czaplicka, M. (2022). Comparison of the Chemical Composition of Selected Varieties of Elderberry with Wild Growing Elderberry. Molecules, 27(16), 5050. https://doi.org/10.3390/molecules27165050