Synthesis, Anticancer Activities and Molecular Docking Studies of a Novel Class of 2-Phenyl-5,6,7,8-tetrahydroimidazo [1,2-b]pyridazine Derivatives Bearing Sulfonamides
Abstract
:1. Introduction
2. Results
2.1. Chemistry
2.2. Antitumor Activity
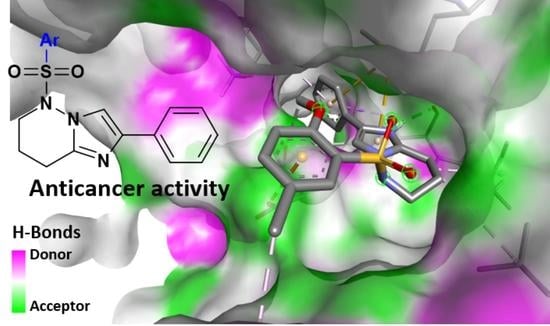
2.3. Molecular Docking Studies
3. Materials and Methods
3.1. General Procedures
3.2. Synthesis and Characterization
3.3. Biology
3.4. Molecular Docking
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Sample Availability
References
- Ur Rashid, H.; Xu, Y.; Muhammad, Y.; Wang, L.; Jiang, J. Research advances on anticancer activities of matrine and its derivatives: An updated overview. Eur. J. Med. Chem. 2019, 161, 205–238. [Google Scholar] [CrossRef] [PubMed]
- Al-Warhi, T.; Sabt, A.; Elkaeed, E.B.; Eldehna, W.M. Recent advancements of coumarin-based anticancer agents: An up-to-date review. Bioorg. Chem. 2020, 103, 104163. [Google Scholar] [CrossRef] [PubMed]
- Globocan (The Global Cancer Observatory). All Cancers; International Agency for Research on Cancer—WHO: Lyon, France, 2020; Volume 419, pp. 199–200. Available online: https://gco.iarc.fr/today/home (accessed on 10 July 2022).
- Cao, X.; Sun, Z.; Cao, Y.; Wang, R.; Cai, T.; Chu, W.; Hu, W.; Yang, Y. Design, synthesis, and structure–activity relationship studies of novel fused heterocycles-linked triazoles with good activity and water solubility. J. Med. Chem. 2014, 57, 3687–3706. [Google Scholar] [CrossRef]
- Feng, Z.; Lu, X.; Gan, L.; Zhang, Q.; Lin, L. Xanthones, a promising anti-inflammatory scaffold: Structure, activity, and drug likeness analysis. Molecules 2020, 25, 598. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Ma, Y.; Cherukupalli, S.; Tavis, J.E.; Menéndez-Arias, L.; Liu, X.; Zhan, P. Discovery and optimization of benzenesulfonamides-based hepatitis B virus capsid modulators via contemporary medicinal chemistry strategies. Eur. J. Med. Chem. 2020, 206, 112714. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Long, J.; Gao, H.; Tang, Z. 2-Aminothiazole: A privileged scaffold for the discovery of anti-cancer agents. Eur. J. Med. Chem. 2021, 210, 112953. [Google Scholar] [CrossRef]
- Boujdi, K.; El Brahmi, N.; Graton, J.; Dubreuil, D.; Collet, S.; Mathé-Allainmat, M.; Akssira, M.; Lebreton, J.; El Kazzouli, S. A regioselective C7 bromination and C7 palladium-catalyzed Suzuki-Miyaura cross-coupling arylation of 4-substituted NH-free indazoles. RSC Adv. 2021, 11, 7107–7114. [Google Scholar] [CrossRef]
- El Abbouchi, A.; Koubachi, J.; El Brahmi, N.; Kazzouli, S. Direct arylation and Suzuki-Miyaura coupling of imidazo [1,2-a]pyridines catalyzed by (SIPr)Pd(allyl)Cl complex under microwave-irradiation. Mediterr. J. Chem. 2019, 9, 347–354. [Google Scholar] [CrossRef]
- Faarasse, S.; El Kazzouli, S.; Bourzikat, O.; Bourg, S.; Aci-Sèche, S.; Bonnet, P.; Suzenet, F.; Guillaumet, G. Palladium-catalyzed regioselective C−H arylation of 4-azaindazole at C3, C5 and C7 positions. Adv. Synth. Catal. 2021, 363, 3937–3945. [Google Scholar] [CrossRef]
- Gambouz, K.; El Abbouchi, A.; Nassiri, S.; Suzenet, F.; Bousmina, M.; Akssira, M.; Guillaumet, G.; El Kazzouli, S. “On Water” palladium catalyzed direct arylation of 1H-indazole and 1H-7-azaindazole. Molecules 2020, 25, 2820. [Google Scholar] [CrossRef]
- Gambouz, K.; El Abbouchi, A.; Nassiri, S.; Suzenet, F.; Bousmina, M.; Akssira, M.; Guillaumet, G.; El Kazzouli, S. Palladium-catalyzed oxidative arylation of 1H-indazoles with arenes. Eur. J. Org. Chem. 2020, 2020, 7435–7439. [Google Scholar] [CrossRef]
- Naas, M.; El Kazzouli, S.; Essassi, E.M.; Bousmina, M.; Guillaumet, G. Palladium-catalyzed oxidative direct C3-and C7-alkenylations of indazoles: Application to the synthesis of Gamendazole. Org. Lett. 2015, 17, 4320–4323. [Google Scholar] [CrossRef] [PubMed]
- Rakesh, K.P.; Wang, S.-M.; Leng, J.; Ravindar, L.; Asiri, A.M.; Marwani, H.M.; Qin, H.-L. Recent development of sulfonyl or sulfonamide hybrids as potential anticancer agents: A key review. Anti-Cancer Agents Med. Chem. 2018, 18, 488–505. [Google Scholar] [CrossRef]
- Bano, S.; Javed, K.; Ahmad, S.; Rathish, I.G.; Singh, S.; Alam, M.S. Synthesis and biological evaluation of some new 2-pyrazolines bearing benzene sulfonamide moiety as potential anti-inflammatory and anti-cancer agents. Eur. J. Med. Chem. 2011, 46, 5763–5768. [Google Scholar] [CrossRef] [PubMed]
- Chalkha, M.; Akhazzane, M.; Moussaid, F.Z.; Daoui, O.; Nakkabi, A.; Bakhouch, M.; Chtita, S.; Elkhattabi, S.; Housseini, A.I.; El Yazidi, M. Design, synthesis, characterization, in vitro screening, molecular docking, 3D-QSAR, and ADME-Tox investigations of novel pyrazole derivatives as antimicrobial agents. New J. Chem. 2022, 46, 2747–2760. [Google Scholar] [CrossRef]
- Chalkha, M.; El Moussaoui, A.; Ben Hadda, T.; Berredjem, M.; Bouzina, A.; Almalki, F.A.; Saghrouchni, H.; Bakhouch, M.; Saadi, M.; El Ammari, L. Crystallographic study, biological evaluation and DFT/POM/Docking analyses of pyrazole linked amide conjugates: Identification of antimicrobial and antitumor pharmacophore sites. J. Mol. Struct. 2022, 1252, 131818. [Google Scholar] [CrossRef]
- Chandna, N.; Kumar, S.; Kaushik, P.; Kaushik, D.; Roy, S.K.; Gupta, G.K.; Jachak, S.M.; Kapoor, J.K.; Sharma, P.K. Synthesis of novel celecoxib analogues by bioisosteric replacement of sulfonamide as potent anti-inflammatory agents and cyclooxygenase inhibitors. Bioorg. Med. Chem. 2013, 21, 4581–4590. [Google Scholar] [CrossRef]
- Ghorab, M.M.; Alsaid, M.S.; El-Gaby, M.S.A.; Safwat, N.A.; Elaasser, M.M.; Soliman, A.M. Biological evaluation of some new N-(2,6-dimethoxypyrimidinyl) thioureido benzenesulfonamide derivatives as potential antimicrobial and anticancer agents. Eur. J. Med. Chem. 2016, 124, 299–310. [Google Scholar] [CrossRef]
- Konda, S.; Raparthi, S.; Bhaskar, K.; Munaganti, R.K.; Guguloth, V.; Nagarapu, L.; Akkewar, D.M. Synthesis and antimicrobial activity of novel benzoxazine sulfonamide derivatives. Bioorg. Med. Chem. Lett. 2015, 25, 1643–1646. [Google Scholar] [CrossRef]
- Naim, M.J.; Alam, O.M.; Alam, J.; Hassan, M.Q.; Siddiqui, N.; Naidu, V.G.M.; Alam, M.I. Design, synthesis and molecular docking of thiazolidinedione based benzene sulphonamide derivatives containing pyrazole core as potential anti-diabetic agents. Bioorg. Chem. 2018, 76, 98–112. [Google Scholar] [CrossRef]
- Ning, X.; Guo, Y.; Ma, X.; Zhu, R.; Tian, C.; Zhang, Z.; Wang, X.; Ma, Z.; Liu, J. Design, synthesis and pharmacological evaluation of (E)-3, 4-dihydroxy styryl sulfonamides derivatives as multifunctional neuroprotective agents against oxidative and inflammatory injury. Bioorg. Med. Chem. 2013, 21, 5589–5597. [Google Scholar] [CrossRef]
- Stokes, S.S.; Albert, R.; Buurman, E.T.; Andrews, B.; Shapiro, A.B.; Green, O.M.; McKenzie, A.R.; Otterbein, L.R. Inhibitors of the acetyltransferase domain of N-acetylglucosamine-1-phosphate-uridylyltransferase/glucosamine-1-phosphate-acetyltransferase (GlmU). Part 2: Optimization of physical properties leading to antibacterial aryl sulfonamides. Bioorg. Med. Chem. Lett. 2012, 22, 7019–7023. [Google Scholar] [CrossRef] [PubMed]
- Ashkenazi, A.; Fairbrother, W.J.; Leverson, J.D.; Souers, A.J. From basic apoptosis discoveries to advanced selective BCL-2 family inhibitors. Nat. Rev. Drug Discov. 2017, 16, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.C.S.; Chan, A.H.Y.; Ganesan, A. Thirty years of HDAC inhibitors: 2020 insight and hindsight. J. Med. Chem. 2020, 63, 12460–12484. [Google Scholar] [CrossRef] [PubMed]
- Yap, J.L.; Chen, L.; Lanning, M.E.; Fletcher, S. Expanding the cancer arsenal with targeted therapies: Disarmament of the antiapoptotic Bcl-2 proteins by small molecules: Miniperspective. J. Med. Chem. 2017, 60, 821–838. [Google Scholar] [CrossRef]
- Zhao, C.; Rakesh, K.P.; Ravidar, L.; Fang, W.-Y.; Qin, H.-L. Pharmaceutical and medicinal significance of sulfur (SVI)-Containing motifs for drug discovery: A critical review. Eur. J. Med. Chem. 2019, 162, 679–734. [Google Scholar] [CrossRef]
- Pezhman, S. Novel hybrid molecules based on triazole-β-lactam as potential biological agents. Mini Rev. Med. Chem. 2021, 21, 536–553. [Google Scholar] [CrossRef]
- Cicenas, J.; Zalyte, E.; Bairoch, A.; Gaudet, P. Kinases and cancer. Cancers 2018, 10, 63. [Google Scholar] [CrossRef]
- El Abbouchi, A.; El Brahmi, N.; Hiebel, M.-A.; Bignon, J.; Guillaumet, G.; Suzenet, F.; El Kazzouli, S. Synthesis and evaluation of a novel class of ethacrynic acid derivatives containing triazoles as potent anticancer agents. Bioorg. Chem. 2021, 115, 105293. [Google Scholar] [CrossRef]
- El Abbouchi, A.; El Brahmi, N.; Hiebel, M.-A.; Bignon, J.; Guillaumet, G.; Suzenet, F.; El Kazzouli, S. Synthesis and biological evaluation of ethacrynic acid derivatives bearing sulfonamides as potent anti-cancer agents. Bioorg. Med. Chem. Lett. 2020, 30, 127426. [Google Scholar] [CrossRef]
- Mignani, S.; El Brahmi, N.; El Kazzouli, S.; Eloy, L.; Courilleau, D.; Caron, J.; Bousmina, M.M.; Caminade, A.-M.; Cresteil, T.; Majoral, J.-P. A novel class of ethacrynic acid derivatives as promising drug-like potent generation of anticancer agents with established mechanism of action. Eur. J. Med. Chem. 2016, 122, 656–673. [Google Scholar] [CrossRef] [PubMed]
- El Brahmi, N.; Mignani, S.M.; Caron, J.; El Kazzouli, S.; Bousmina, M.M.; Caminade, A.-M.; Cresteil, T.; Majoral, J.-P. Investigations on dendrimer space reveal solid and liquid tumor growth-inhibition by original phosphorus-based dendrimers and the corresponding monomers and dendrons with ethacrynic acid motifs. Nanoscale 2015, 7, 3915–3922. [Google Scholar] [CrossRef] [PubMed]
- El Kazzouli, S.; Zyad, A.; El Brahmi, N.; El Abbouchi, A.; Boujdi, K.; Bousmina, M.; Ait Mouse, H.; Tilaoui, M. Antitumour Activities of a Novel Family of Ethacrynic Acid Derivatives. U.S. Patent 0024957 A1, 27 January 2022. [Google Scholar]
- El Akkaoui, A.; Koubachi, J.; El Kazzouli, S.; Berteina-Raboin, S.; Mouaddib, A.; Guillaumet, G. Efficient and regioselective functionalization of imidazo [1, 2-b] pyridazines via palladium-catalyzed cross-coupling reaction and SNAr. Tetrahedron Lett. 2008, 49, 2472–2475. [Google Scholar] [CrossRef]
- Yoneda, F.; Ohtaka, T.; Nitta, Y. Pyridazin-derivate. VI. Synthese der derivate des imidazo [1, 2-b] pyridazins. Chem. Pharm. Bull. 1964, 12, 1351–1356. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Tan, M.; Yu, D. Molecular mechanisms of ErbB2-mediated breast cancer chemoresistance. In Breast Cancer Chemosensitivity. Advances in Experimental Medicine and Biology; Yu, D., Hung, M.C., Eds.; Springer: New York, NY, USA, 2007; Volume 608, pp. 119–129. [Google Scholar] [CrossRef]
- Richardsen, E.; Uglehus, R.D.; Johnsen, S.H.; Busund, L.-T. Macrophage-colony stimulating factor (CSF1) predicts breast cancer progression and mortality. Anticancer Res. 2015, 35, 865–874. [Google Scholar]
- Liu, D.; Zhou, K. BRAF/MEK pathway is associated with breast cancer in ER-dependent mode and improves ER status-based cancer recurrence prediction. Clin. Breast Cancer 2020, 20, 41–50. [Google Scholar] [CrossRef]
- Heldin, C.-H. Targeting the PDGF signaling pathway in tumor treatment. Cell Commun. Signal. 2013, 11, 97. [Google Scholar] [CrossRef]
- Alqathama, A. BRAF in malignant melanoma progression and metastasis: Potentials and challenges. Am. J. Cancer Res. 2020, 10, 1103–1114. [Google Scholar]
- Dhomen, N.; Marais, R. BRAF signaling and targeted therapies in melanoma. Hematol. Clin. 2009, 23, 529–545. [Google Scholar] [CrossRef]
- Pham, D.D.M.; Guhan, S.; Tsao, H. KIT and melanoma: Biological insights and clinical implications. Yonsei Med. J. 2020, 61, 562–571. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, K.E.; McArthur, G.A. The cell-cycle regulator CDK4: An emerging therapeutic target in melanoma. Clin. Cancer Res. 2013, 19, 5320–5328. [Google Scholar] [CrossRef] [PubMed]


 | ||||
|---|---|---|---|---|
| Entry | Tosyl Chloride (Equiv.) | Base (2 Equiv.) | Time (h) | Yield% |
| 1 | 2 | Et3N | 48 | 68 |
| 2 | 2 | DIPEA | 48 | 55 |
| 3 | 2 | Pyridine | 1 | 87 |
| 4 | 1.1 | Pyridine | 1 | 86 |
| Compounds | Human Cancer Cell Lines IC50 (μM) a | |||||
|---|---|---|---|---|---|---|
| A549 b | HS-683 c | MCF-7 d | SK-MEL-28 e | B16-F1 f | c Log P g | |
| 4a | 10–100 | >100 | >100 | >100 | 10–100 | 3.7 |
| 4b | >100 | >100 | >100 | >100 | >100 | 3.1 |
| 4c | 10–100 | >100 | 10–100 | >100 | >100 | 2.9 |
| 4d | >100 | 10–100 | 10–100 | >100 | 10–100 | 3.0 |
| 4e | >100 | >100 | 9.4 | >100 | >100 | 4.0 |
| 4f | >100 | 67.9 | 96.5 | 7.8 | 10.8 | 4.1 |
| 4g | >100 | >100 | >100 | 10–50 | >100 | 4.4 |
| 5-FU | 1.2 | 4.3 | 2.3 | 3.3 | 0.3 | |
| Etoposide | 0.9 | 0.8 | 3.3 | 1.1 | 1.3 | |
| 4e | ||
| BRAF |  |  |
| CSF1R |  |  |
| ErbB2 |  |  |
| MEK2 |  |  |
| 4f | ||
| 2D diagram | ||
| PDGFRA |  |  |
| BRAF |  |  |
| MEK2 |  |  |
| KIT |  |  |
| CDK4 |  |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourzikat, O.; El Abbouchi, A.; Ghammaz, H.; El Brahmi, N.; El Fahime, E.; Paris, A.; Daniellou, R.; Suzenet, F.; Guillaumet, G.; El Kazzouli, S. Synthesis, Anticancer Activities and Molecular Docking Studies of a Novel Class of 2-Phenyl-5,6,7,8-tetrahydroimidazo [1,2-b]pyridazine Derivatives Bearing Sulfonamides. Molecules 2022, 27, 5238. https://doi.org/10.3390/molecules27165238
Bourzikat O, El Abbouchi A, Ghammaz H, El Brahmi N, El Fahime E, Paris A, Daniellou R, Suzenet F, Guillaumet G, El Kazzouli S. Synthesis, Anticancer Activities and Molecular Docking Studies of a Novel Class of 2-Phenyl-5,6,7,8-tetrahydroimidazo [1,2-b]pyridazine Derivatives Bearing Sulfonamides. Molecules. 2022; 27(16):5238. https://doi.org/10.3390/molecules27165238
Chicago/Turabian StyleBourzikat, Otmane, Abdelmoula El Abbouchi, Hamza Ghammaz, Nabil El Brahmi, Elmostfa El Fahime, Arnaud Paris, Richard Daniellou, Franck Suzenet, Gérald Guillaumet, and Saïd El Kazzouli. 2022. "Synthesis, Anticancer Activities and Molecular Docking Studies of a Novel Class of 2-Phenyl-5,6,7,8-tetrahydroimidazo [1,2-b]pyridazine Derivatives Bearing Sulfonamides" Molecules 27, no. 16: 5238. https://doi.org/10.3390/molecules27165238
APA StyleBourzikat, O., El Abbouchi, A., Ghammaz, H., El Brahmi, N., El Fahime, E., Paris, A., Daniellou, R., Suzenet, F., Guillaumet, G., & El Kazzouli, S. (2022). Synthesis, Anticancer Activities and Molecular Docking Studies of a Novel Class of 2-Phenyl-5,6,7,8-tetrahydroimidazo [1,2-b]pyridazine Derivatives Bearing Sulfonamides. Molecules, 27(16), 5238. https://doi.org/10.3390/molecules27165238