Sorption Preconcentration and Analytical Determination of Cu, Zr and Hf in Waste Samarium–Cobalt Magnet Samples
Abstract
:1. Introduction
2. Materials and Methods
2.1. Apparatus
2.2. Reagents and Materials
2.3. Synthesis of Sorbents
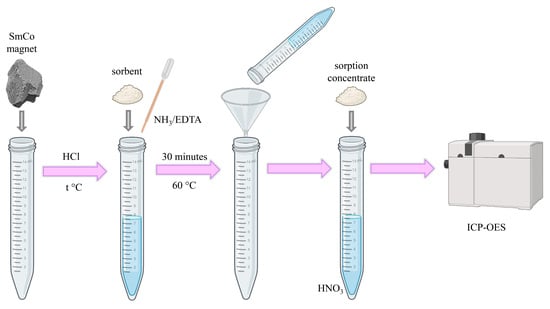
2.4. Sample Preparation
2.5. Sorption Procedure
3. Results and Discussion
3.1. Characterisation of Sorbents
3.2. Sorption without Additional Reagents
3.3. Sorption in an Acidic Environment
3.4. Introduction of Additional Sorption Reagents
3.5. The Effect of Reagent Concentration
3.6. The Effect of Sample Acidity
3.7. The Study of Kinetics
3.8. The Effect of Temperature
3.9. Influence of Adsorbent Dose
3.10. The Matrix Effect
3.11. Analytical Performance
3.12. Analysis Application
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhou, X.; Huang, A.; Cui, B.; Sutherland, J.W. Techno-Economic Assessment of a Novel SmCo Permanent Magnet Manufacturing Method. Procedia CIRP 2021, 98, 127–132. [Google Scholar] [CrossRef]
- Li, X.; Orefice, Z.; Li, M.; Binnemans, K. Metal Recovery from Spent Samarium−Cobalt Magnets Using a Trichloride Ionic Liquid. ACS Sustain. Chem. Eng. 2019, 7, 2578–2584. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, T.; Bertau, M. Recycling of rare earth elements from SmCo5-Magnets via solid-state chlorination. J. Clean. Prod. 2019, 246, 118980. [Google Scholar] [CrossRef]
- Avdibegović, D.; Binnemans, K. Chromatographic separation of rare earths from aqueous and ethanolic leachates of NdFeB and SmCo magnets by a supported ionic liquid phase. RSC Adv. 2021, 11, 8207–8217. [Google Scholar] [CrossRef]
- Silva de Souza Lima Cano, N.; Iacovidou, E.; Rutkowski, E.W. Typology of Municipal Solid Waste Recycling Value Chains: A Global Perspective. J. Clean. Prod. 2022, 336, 130386. [Google Scholar] [CrossRef]
- Papai, R.; Sousa de Freitas, M.A.; Torre da Fonseca, K.; Alves de Almeida, G.; Filipini da Silveira, J.R.; Nunis da Silva, A.L.; Ferreira Neto, J.B.; Lino dos Santos, C.A.; Gomes Landgraf, F.J.; Luz, M.S. Additivity of optical emissions applied to neodymium and praseodymium quantification in metallic didymium and (Nd.Pr)-Fe-B alloy samples by low-resolution atomic emission spectrometry: An evaluation of the mathematical approach used to solve spectral interferences. Anal. Chim. Acta 2019, 1085, 21–28. [Google Scholar] [CrossRef]
- München, D.D.; Veit, H.M. Neodymium as the main feature of permanent magnets from hard disk drives (HDDs). Waste Manag. 2017, 61, 372–376. [Google Scholar] [CrossRef]
- Renko, M.; Osojnik, A.; Hudnik, V. ICP emission spectrometric analysis of rare earth elements in permanent magnet alloys. Fresenius J. Anal. Chem. 1995, 351, 610–613. [Google Scholar] [CrossRef]
- Chausseau, M.; Stankova, A.; Li, Z.; Hunault, P.; Savadkouei, H. High-resolution ICP-OES for the determination of trace elements in a rare earth element matrix and in NdFeB magnetic materials. Spectroscopy 2014, 29, 30–41. [Google Scholar]
- Iwasaki, K.; Uchida, H. Rapid determination of Major and Minor Elements in Rare Earth-Cobalt Magnets by Inductively Coupled Plasma Atomic Emission Spectrometry. Anal. Sci. 1986, 2, 261–264. [Google Scholar] [CrossRef]
- Kuchumov, V.A.; Shoomkin, S.S. Analysis of the chemical composition of the bearing alloy used in the production of Sm-Co-based permanent magnets. St. Petersburg Polytech. Univ. J. Eng. Sci. Technol. 2017, 23, 219–225. [Google Scholar] [CrossRef]
- Petrova, K.V.; Baranovskaya, V.B.; Korotkova, N.A. Direct inductively coupled plasma optical emission spectrometry for analysis of waste samarium–cobalt magnets. Arab. J. Chem. 2022, 15, 103501. [Google Scholar] [CrossRef]
- Orefice, M.; Audoor, H.; Li, Z.; Binnemans, K. Solvometallurgical route for the recovery of Sm, Co, Cu and Fe from SmCo permanent magnets. Sep. Purif. Technol. 2019, 219, 281–289. [Google Scholar] [CrossRef]
- Xu, X.; Khoshima, S.; Karajic, M.; Balderman, J.; Markovic, K.; Scancar, J.; Rozman, K.Z. Electrochemical routes for environmentally friendly recycling of rare-earth-based (Sm–Co) permanent magnets. J. Appl. Electrochem. 2022, 52, 1081–1090. [Google Scholar] [CrossRef]
- Riano, S.; Binnemans, K. Extraction and separation of neodymium and dysprosium from used NdFeB magnets: An application of ionic liquids in solvent extraction towards the recycling of magnets. Green Chem. 2015, 17, 2931–2942. [Google Scholar] [CrossRef]
- Ni, A.C.; Wang, A.Y.; Chen, S.; Chang, G.; You, S. Simultaneous recovery of rare earth elements from waste permanent magnets (WPMs) leach liquor by solvent extraction and hollow fiber supported liquid membrane. Chem. Eng. Process 2020, 148, 107831. [Google Scholar]
- Yamada, E.; Murakami, H.; Nishihama, S.; Yoshizuka, K. Separation process of dysprosium and neodymium from waste neodymium magnet. Sep. Purif. Technol. 2018, 192, 62–68. [Google Scholar] [CrossRef]
- Zheng, X.; Liu, E.; Zhang, F.; Yan, Y.; Pan, J. Efficient adsorption and separation of dysprosium from NdFeB magnets in an acidic system by ion imprinted mesoporous silica sealed in a dialysis bag. Green Chem. 2016, 18, 5031–5040. [Google Scholar] [CrossRef]
- Rodrigues, D.G.; Monge, S.; Pellet-Rostaing, S.; Dacheux, N.; Bouyer, D.; Faur, C. A new carbamoyl methylphosphonic acid-based polymer for the selective sorption of rare earth elements. Chem. Eng. J. 2019, 371, 857–867. [Google Scholar] [CrossRef]
- Dudarko, O.; Kobylinska, N.; Kessler, V.; Seisenbaeva, G. Recovery of rare earth elements from NdFeB magnet by mono-and bifunctional mesoporous silica: Waste recycling strategies and perspectives. Hydrometallurgy 2022, 210, 105855. [Google Scholar] [CrossRef]
- Filatova, D.G.; Arkhipenko, A.A.; Statkus, M.A.; Es’kina, V.V.; Baranovskaya, V.B.; Karpov, Y.A. Sorption of Se(IV) from Aqueous Solutions with Subsequent Determination by X-Ray Fluorescence Analysis. Inorg. Mater. 2021, 57, 1427–1430. [Google Scholar] [CrossRef]
- Eskina, V.V.; Dalnova, O.A.; Kareva, E.N.; Baranovskaya, V.B.; Karpov, Y.A. Determination of impurities in high-purity niobium (V) oxide by high-resolution continuum source graphite furnace atomic absorption spectrometry after sorption preconcentration. J. Anal. Chem. 2017, 72, 649–655. [Google Scholar] [CrossRef]
- Dalnova, O.A.; Baranovskaya, V.B.; Dalnova, Y.S.; Karpov, Y.A. New Complexing Polymer Aminothioether Sorbents in the Analytical Control of Recyclable Metal-Containing Raw Material of Rare and Noble Metals. J. Anal. Chem. 2018, 73, 221–227. [Google Scholar] [CrossRef]
- Eskina, V.V.; Baranovskaya, V.B.; Karpov, Y.A.; Dalnova, O.A.; Filatova, D.G. Separation And Preconcentration of Platinum-Group Metals From Spent Autocatalysts Solutions Using A Hetero-Polymeric S, N-Containing Sorbent And Determination By High-Resolution Continuum Source Graphite Furnace Atomic Absorption Spectrometry. Talanta 2016, 159, 103–110. [Google Scholar] [CrossRef]
- Dalnova, O.A.; Eskina, V.V.; Dalnova, Y.S.; Rubtsov, V.N.; Skripnikov, V.N.; Shevchenko, E.V. Method for Obtaining Sorbent for Recovering Selenium, Tellurium. U.S. Patent 4,586,960, 5 July 2018. [Google Scholar]
- Dalnova, O.A. Sorption-Atom-Absorption Analysis of Secondary and Man-Made Raw Materials for Platinum Metal Content. Ph.D. Thesis, Institute of Rare-Metal Industry Giredmet, Moscow, Russia, 3 December 2009. [Google Scholar]
- Nakanishi, K.; Solomon, P.H. Infrared Absorption Spectroscopy, 2nd ed.; Holden-Day, Inc.: San Francisco, CA, USA, 1977. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Affolter, C. Structure Determination of Organic Compounds; Springer: Berlin/Heidelberg, Germany, 2000; p. 421. [Google Scholar]
- Nowack, B.; Sigg, L. Adsorption of EDTA and Metal–EDTA Complexes onto Goethite. J. Colloid Interface Sci. 1996, 177, 106–121. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Austruy, A.; Echevarria, G.; Arshad, M.; Sanaullah, M.; Aslam, M.; Nadeem, M.; Nasim, W.; Dumat, C. EDTA-Enhanced Phytoremediation of Heavy Metals: A Review. Soil Sediment Contam. Int. J. 2014, 23, 389–416. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Liu, Y.; Ren, J.; Zhang, Y.; Qu, G.; Wang, T. Effective removal of the heavy metal-organic complex Cu-EDTA from water by catalytic persulfate oxidation: Performance and mechanisms. J. Clean. Prod. 2021, 314, 128119. [Google Scholar] [CrossRef]
- Gao, X.; Zheng, H.; Shang, G.; Xu, J. Colorimetric detection of fluoride in an aqueous solution using Zr(IV)–EDTA complex and a novel hemicyanine dye. Talanta 2007, 73, 770–775. [Google Scholar] [CrossRef]
- Das, S.K.; Tomar, B.S.; Prakash, S. Perturbed angular correlation studies of the Hf—EDTA complex. Polyhedron 1990, 9, 2705–2708. [Google Scholar] [CrossRef]
- Peng, C.; Chai, L.; Tang, C.; Min, X.; Song, Y.; Duan, C.; Yu, C. Study on the mechanism of copper–ammonia complex decomposition in struvite formation process and enhanced ammonia and copper removal. J. Environ. Sci. 2017, 51, 222–233. [Google Scholar] [CrossRef]
- Luo, L.; Liu, Y.; Wu, Z.; Liu, J.; Cao, X.; Lin, J.; Ling, R.; Luo, X.; Wang, C. Macromolecular-metal complexes induced by Co(II) with polymer and flexible ligands for ammonia uptake compared with MOFs. Chem. Eng. J. 2022, 448, 137626. [Google Scholar] [CrossRef]
- Mousavi, S.V.; Ahranjani, P.J.; Saei, S.F.; Mehrdadi, N.; Bidhendi, G.N.; Jume, B.H.; Rezania, S.; Mojiri, A. Ammonia removal from industrial effluent using zirconium oxide and graphene-oxide nanocomposites. Chemosphere 2022, 297, 134008. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, K.L.; Bell, P.B.; Watson, R.E. Dinuclear excited-state complexes from UV laser excitation of aqueous ammonia, methylamine, and ethylamine copper(I) complexes. Coord. Chem. Rev. 2002, 229, 133–146. [Google Scholar] [CrossRef]
- Nguyen, H.V.L.; Gulaczyk, I.; Kręglewski, M.; Kleiner, I. Large amplitude inversion tunneling motion in ammonia, methylamine, hydrazine, and secondary amines: From structure determination to coordination chemistry. Coord. Chem. Rev. 2021, 436, 213797. [Google Scholar] [CrossRef]
- Shestakova, E.P.; Varshavsky, Y.S.; Khrustalev, V.N.; Smirnov, S.N. Synthesis and characterization of acetamidine rhodium(III) cationic methyl complex, trans-[Rh(Acac)(PPh3)2(CH3){NHC(NH2)CH3}][BPh4], produced by ammination of acetonitrile ligand in trans-[Rh(Acac)(PPh3)2(CH3)(CH3CN)][BPh4]. J. Organomet. Chem. 2013, 735, 47–51. [Google Scholar] [CrossRef]
- Amekraz, B.; Tortajada, J.; Morizur, J.-P.; González, A.I.; Mó, O.; Yáñez, M. Acetamidine-Mg+(2S) complexes; the performance of different exchange and correlation functionals. J. Mol. Struct. THEOCHEM 1996, 371, 313–324. [Google Scholar] [CrossRef]
- Hynes, M.J.; Wurm, K.; Moloney, A. Reduction of the bis(pentane-2,4-dionato)diaquo manganese(III) complex by hydroxylamine and L-ascorbic acid in aqueous solution. Inorg. Chim. Acta 1993, 211, 5–10. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Wang, L.; Liu, Y.-L.; Zhao, Q.; Ma, J. Unraveling the interaction of hydroxylamine and Fe(III) in Fe(II)/Persulfate system: A kinetic and simulating study. Water Res. 2020, 168, 115093. [Google Scholar] [CrossRef]
- Mejri, A.; Assili, K.; Alouani, K. Electrochemical and spectroscopic characterization of zinc (II) complex with Bis(tetraethylthiophosphoramidoyl)methylamine. J. Electroanal. Chem. 2016, 767, 134–140. [Google Scholar] [CrossRef]
- Jeong, G.H.; Kim, Y.; Seff, K. Structure of a methylamine sorption complex of fully dehydrated Cd2+-exchanged zeolite X, ∣Cd46(CH3NH2)16∣[Si100Al92O384]-FAU. Microporous Mesoporous Mater. 2006, 90, 16–22. [Google Scholar] [CrossRef]
- Aroney, M.J.; Clarkson, R.M.; Hambley, T.W.; Pierens, R.K. A study of polarities, polarisabilities and infrared spectra of LM(CO)5 complexes (M=Cr, Mo or W.; L = trimethylamine, quinuclidine, pyridine, p-methyl- or p-t-butylpyridine) and of the X-ray crystal structure of quinuclidine-Cr(CO)5: Evidence for π-acceptor behaviour of coordinated pyridine. J. Organomet. Chem. 1992, 426, 331–342. [Google Scholar] [CrossRef]
- Millington, K.R.; Wade, S.R.; Willey, G.R.; Drew, M.G.B. Zinc(II), iron(III), molybdenum(II) chloride and molybdenum(V), molybdenum(VI) oxochloride complexes of trimethylamine: Synthesis, spectra and X-ray crystal structure characterization. Inorg. Chim. Acta 1984, 89, 185–191. [Google Scholar] [CrossRef]
- Hughes, J.; Willey, G.R. Trimethylamine complexes of zirconium(IV) halides. Inorg. Chim. Acta 1976, 20, 137–140. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, X.; Zhang, W.; Liu, X.; Liu, Q. Fluorescent Detector for NH3 based on Responsive Europium(III)–Salicylic acid Complex Hydrogels. J. Photochem. Photobiol. A Chem. 2021, 404, 112901. [Google Scholar] [CrossRef]
- Nurchi, V.M.; Crespo-Alonso, M.; Toso, L.; Lachowicz, J.I.; Crisponi, G.; Alberti, G.; Biesuz, R.; Domínguez-Martín, A.; Niclós-Gutíerrez, J.; González-Pérez, J.M.; et al. Iron(III) and aluminium(III) complexes with substituted salicyl-aldehydes and salicylic acids. J. Inorg. Biochem. 2013, 128, 174–182. [Google Scholar] [CrossRef]
- Cabeza, G.; Contreras, B.; Araujo, M.L.; Brito, F.; Hernandez, L.; Pérez, A.; Carpio, E.D.; Lubes, V. Stability constants of the ternary complexes formed between vanadium(III)–salicylic acid and amino acids. J. Mol. Liq. 2015, 207, 323–326. [Google Scholar] [CrossRef]
- Rojas, D.; Araujo, M.L.; Martínez, J.D.; Brito, F.; Carpio, E.D.; Reina, K.; Landaeta, V.R.; Hernández, L.; Lubes, V. Mixed-ligand complex formation equilibria of copper(II), salicylic acid and some amino acids. J. Mol. Liq. 2016, 220, 238–242. [Google Scholar] [CrossRef]
- Cornell, N.W.; Crivaro, K.E. Stability constant for the zinc-dithiothreitol complex. Anal. Biochem. 1972, 47, 203–208. [Google Scholar] [CrossRef]











| Forward power, W | 900–1400 |
| Coolant gas flow, L/min | 15 |
| Auxiliary gas flow, L/min | 0.35 |
| Nebuliser gas flow, L/min | 0.50–1.3 |
| Sample flow rate, rpm | 60 |
| Pump tube, mm | 0.64 |
| Radial viewing height, mm | 10 |
| Injector diameter, mm | 2 |
| Pneumatic nebuliser | SeaSpray nebuliser, glass expansion |
| Spray chamber | Cyclonic spray chamber, glass expansion |
| Wavelength (nm) | Cu 223.008, Cu 224.700, Cu 323.452, Ti 337.280, Zr 267.863, Zr 339.198, Nb 295.088, Nb 309.418, Nb 264.141, Hf 277.336, Hf 282.022 |
| Forward power/W | 1300 |
| Coolant gas flow/L·min−1 | 0.8 |
| Auxiliary gas flow/L·min−1 | 13 |
| Nebuliser gas flow/L·min−1 | 0.85–0.90 |
| Sample flow rate/rpm | 50 |
| Sampling depth/relative units | 101 |
| Potential at the extractor lens/V | −400 |
| Spray booth temperature/°C | 3 |
| Level of oxide ions/% | <2 |
| Level of doubly charged ions/% | <1.5 |
| Measurement mode | Peak hopping |
| Pneumatic nebuliser | SeaSpray Nebuliser, glass expansion |
| Spray chamber | Quartz conical, Peltier cooled |
| Isotopes of elements to be determined/m/z | 47Ti, 63Cu, 91Zr, 177Hf, 93Nb |
| Element | RM 1 (mg/L) | RM 2 (mg/L) | RM 3 (mg/L) | RM 4 (mg/L) |
|---|---|---|---|---|
| Cu | 0.10 ± 0.01 | 0.50 ± 0.02 | 0.49 ± 0.02 | 2.41 ± 0.09 |
| Zr | 0.07 ± 0.01 | 0.15 ± 0.02 | 0.040 ± 0.01 | 1.01 ± 0.05 |
| Hf | 0.25 ± 0.02 | 0.10 ± 0.01 | 0.27 ± 0.02 | 0.29 ± 0.02 |
| Element | PTE | PED | TDA | PhED |
|---|---|---|---|---|
| Ti | 7 | 19 | 17 | 16 |
| Cu | 56 | 76 | 63 | 61 |
| Zr | 14 | 20 | 17 | 18 |
| Nb | 25 | 36 | 38 | 27 |
| Hf | 20 | 22 | 23 | 21 |
| Element | HCl | HNO3 | ||||||
|---|---|---|---|---|---|---|---|---|
| PTE | PED | TDA | PhED | PTE | PED | TDA | PhED | |
| Ti | 35 | 24 | 37 | 30 | 14 | 21 | 26 | 20 |
| Cu | 91 | 86 | 80 | 61 | 63 | 80 | 74 | 65 |
| Zr | 40 | 31 | 27 | 14 | 18 | 28 | 24 | 21 |
| Nb | 36 | 35 | 42 | 21 | 28 | 38 | 40 | 31 |
| Hf | 29 | 24 | 30 | 17 | 23 | 31 | 28 | 27 |
| Element | Recovery from the Co Matrix, % | Recovery from the Sm Matrix, % |
|---|---|---|
| Ti | 0 | 3 |
| Cu | 87 | 70 |
| Nb | 61 | 60 |
| Zr | 18 | 97 |
| Hf | 11 | 95 |
| Element | RM 1 | RM 2 | ||||
|---|---|---|---|---|---|---|
| Added (mg/L) | Found (mg/L) | Recovery (%) | Added (mg/L) | Found (mg/L) | Recovery (%) | |
| Cu | 0 | 0.12 ± 0.01 | - | 0 | 0.48 ± 0.03 | - |
| 0.5 | 0.65 ± 0.04 | 106 | 0.5 | 0.99 ± 0.08 | 102 | |
| Zr | 0 | 0.09 ± 0.01 | - | 0 | 0.16 ± 0.01 | - |
| 0.5 | 0.58 ± 0.04 | 98 | 0.5 | 0.68 ± 0.04 | 94 | |
| Hf | 0 | 0.22 ± 0.02 | - | 0 | 0.07 ± 0.01 | - |
| 0.5 | 0.70 ± 0.06 | 96 | 0.5 | 0.59 ± 0.03 | 92 | |
| Element | RM 3 (μg/g) | RM 4 (μg/g) | ||||||
|---|---|---|---|---|---|---|---|---|
| ICP-OES with Preconcentration | r | I(TO) | ICP-MS | ICP-OES with Preconcentration | r | I(TO) | ICP-MS | |
| Cu | 0.53 ± 0.04 | 0.056 | 0.067 | 0.48 ± 0.03 | 2.50 ± 0.14 | 0.197 | 0.237 | 2.33 ± 0.13 |
| Zr | 0.038 ± 0.012 | 0.016 | 0.020 | 0.035 ± 0.009 | 1.05 ± 0.08 | 0.113 | 0.135 | 0.99 ± 0.07 |
| Hf | 0.24 ± 0.03 | 0.042 | 0.050 | 0.26 ± 0.02 | 0.25 ± 0.03 | 0.042 | 0.050 | 0.30 ± 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arkhipenko, A.A.; Petrova, K.V.; Baranovskaya, V.B. Sorption Preconcentration and Analytical Determination of Cu, Zr and Hf in Waste Samarium–Cobalt Magnet Samples. Molecules 2022, 27, 5275. https://doi.org/10.3390/molecules27165275
Arkhipenko AA, Petrova KV, Baranovskaya VB. Sorption Preconcentration and Analytical Determination of Cu, Zr and Hf in Waste Samarium–Cobalt Magnet Samples. Molecules. 2022; 27(16):5275. https://doi.org/10.3390/molecules27165275
Chicago/Turabian StyleArkhipenko, Alexandra Alexandrovna, Kseniya Vadimovna Petrova, and Vasilisa Borisovna Baranovskaya. 2022. "Sorption Preconcentration and Analytical Determination of Cu, Zr and Hf in Waste Samarium–Cobalt Magnet Samples" Molecules 27, no. 16: 5275. https://doi.org/10.3390/molecules27165275
APA StyleArkhipenko, A. A., Petrova, K. V., & Baranovskaya, V. B. (2022). Sorption Preconcentration and Analytical Determination of Cu, Zr and Hf in Waste Samarium–Cobalt Magnet Samples. Molecules, 27(16), 5275. https://doi.org/10.3390/molecules27165275