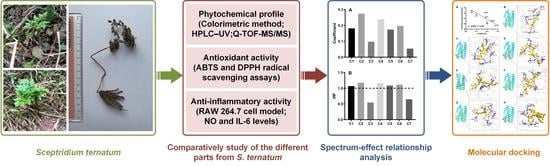
Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Characterization
2.2. Chromatographic Analyses
2.3. Antioxidant Activity
2.4. Anti-Inflammatory Activity
2.5. Spectrum-Effect Relationship Analysis
2.6. Molecular Modeling
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material and S. ternatum Extraction Protocol
3.3. Spectrophotometric Analysis
3.3.1. Determination of Total Phenols
3.3.2. Determination of Total Flavonoids
3.3.3. ABTS Radical Scavenging Assay
3.3.4. DPPH Radical Scavenging Assay
3.4. HPLC–UV and HPLC–ESI-Q-TOF-MS/MS Analyses
3.5. Cell Culture and Cell Viability Assay
3.6. NO Assay and Enzyme-Linked Immunosorbent Assay (ELISA)
3.7. Spectrum-Effect Relationship Analysis
3.8. Compound-Target Molecular Modeling
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular insight into the therapeutic promise of flavonoids against alzheimer’s disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef]
- Lalani, S.; Poh, C.L. Flavonoids as antiviral agents for Enterovirus A71 (EV-A71). Viruses 2020, 12, 184. [Google Scholar] [CrossRef]
- Rufino, A.T.; Costa, V.M.; Carvalho, F.; Fernandes, E. Flavonoids as antiobesity agents: A review. Med. Res. Rev. 2021, 41, 556–585. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Cui, J.; Li, H.; Hasan, B.; Xin, X. Chemical component and in vitro protective effects of Matricaria chamomilla (L.) against lipopolysaccharide insult. J. Ethnopharmacol. 2022, 296, 115471. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, X.; Li, J.; Yin, H.; Liu, F.; Hu, C.; Li, L. Apigenin attenuates acetaminophen-induced hepatotoxicity by activating AMP-activated protein kinase/carnitine palmitoyltransferase I pathway. Front. Pharmacol. 2020, 11, 549057. [Google Scholar] [CrossRef]
- Botrychium Ternatum (Thunb.) Sw. Available online: http://www.iplant.cn/info/Botrychium%20ternatum?t=foc (accessed on 26 June 2022).
- Zou, X.; Wagner, W.H. A preliminary review of Botrychium in China. Am. Fern J. 1988, 78, 122–135. [Google Scholar]
- Yuan, Y.; Yang, B.; Ye, Z.; Zhang, M.; Yang, X.; Xin, C.; Lin, M.; Huang, P. Sceptridium ternatum extract exerts antiasthmatic effects by regulating Th1/Th2 balance and the expression levels of leukotriene receptors in a mouse asthma model. J. Ethnopharmacol. 2013, 149, 701–706. [Google Scholar] [CrossRef]
- Huang, P.; Xin, W.; Zheng, X.; Fang, L.; Li, Q.; Lv, G. Screening of Sceptridium ternatum for antitussive and antiasthmatic activity and associated mechanisms. J. Int. Med. Res. 2017, 45, 1985–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xin, W.X.; Li, Q.L.; Fang, L.; Zhong, L.K.; Zheng, X.W.; Huang, P. Preventive effect and mechanism of ethyl acetate extract of Sceptridium ternatum in monocrotaline-induced pulmonary arterial hypertension. Chin. J. Integr. Med. 2020, 26, 205–211. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.S.; Yun, B.S.; Kyoung, C.H.; Suk, Y.M.; Yeon, K.J. Skin External Composition Containing Sceptridium Ternatum Extract. Korean Patent No. KR-20130099610-A, 6 September 2013. [Google Scholar]
- Lim, D.; Kim, M.K.; Jang, Y.P.; Kim, J. Sceptridium ternatum attenuates allergic contact dermatitis-like skin lesions by inhibiting T helper 2-type immune responses and inflammatory responses in a mouse model. J. Dermatol. Sci. 2015, 79, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Zhong, L.; Kong, S.; Zhang, Y.; Huang, P. Comprehensive evaluation of the antioxidant capacity of Sceptridium ternatum using multiple colorimetric methods and 1,1-diphenyl-2-picrylhydrazyl-high-performance liquid chromatography analysis. J. Sep. Sci. 2020, 43, 3615–3624. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Jiang, N.; Xu, X.; Liu, C.; Liu, Z.; Zhang, Y.; Kang, W. Anti-hepatoma compound determination by the method of spectrum effect relationship, component knock-out, and UPLC-MS2 in Scheflera heptaphylla (L.)Frodin Harms and its mechanism. Front. Pharmacol. 2020, 11, 1342. [Google Scholar] [CrossRef]
- Zhou, X.; Liu, H.; Zhang, M.; Li, C.; Li, G. Spectrum-effect relationship between UPLC fingerprints and anti-lung cancer effect of Panax ginseng. Phytochem. Anal. 2021, 32, 339–346. [Google Scholar] [CrossRef]
- Yin, B.; Bi, Y.M.; Fan, G.J.; Xia, Y.Q. Molecular mechanism of the effect of Huanglian Jiedu Decoction on Type 2 diabetes mellitus based on network pharmacology and molecular docking. J. Diabetes. Res. 2020, 2020, 5273914. [Google Scholar] [CrossRef]
- Zhao, R.; Liu, Y.; Wang, H.; Yang, J.; Niu, W.; Fan, S.; Xiong, W.; Ma, J.; Li, X.; Phillips, J.B.; et al. BRD7 plays an anti-inflammatory role during early acute inflammation by inhibiting activation of the NF-κB signaling pathway. Cell. Mol. Immunol. 2017, 14, 830–841. [Google Scholar] [CrossRef]
- Aoki, Y.; Narazaki, M.; Kishimoto, T.; Tosato, G. Receptor engagement by viral interleukin-6 encoded by Kaposi sarcoma-associated herpesvirus. Blood 2001, 98, 3042–3049. [Google Scholar] [CrossRef]
- Berg, A.F.; Ettich, J.; Weitz, H.T.; Krusche, M.; Floss, D.M.; Scheller, J.; Moll, J.M. Exclusive inhibition of IL-6 trans-signaling by soluble gp130FlyR Fc. Cytokine: X 2021, 3, 100058. [Google Scholar] [CrossRef]
- Xing, L.; Zhang, X.; Zhang, X.; Tong, D. Expression scoring of a small-nucleolar-RNA signature identified by machine learning serves as a prognostic predictor for head and neck cancer. J. Cell. Physiol. 2020, 235, 8071–8084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, W.; Huang, Y.; Chen, S.; Teng, T.; Shi, Y.; Sun, Z.; Xu, Y. Mechanisms of Rhizoma Coptidis against type 2 diabetes mellitus explored by network pharmacology combined with molecular docking and experimental validation. Sci. Rep. 2021, 11, 20849. [Google Scholar] [CrossRef] [PubMed]
- Bazylko, A.; Parzonko, A.; Jeż, W.; Osińska, E.; Kiss, A.K. Inhibition of ROS production, photoprotection, and total phenolic, flavonoids and ascorbic acid content of fresh herb juice and extracts from the leaves and flowers of Tropaeolum majus. Ind. Crops Prod. 2014, 55, 19–24. [Google Scholar]
- Zhu, J.F.; Yi, X.J.; Zhang, J.H.; Chen, S.Q.; Wu, Y.J. Rapid screening of brain-penetrable antioxidants from natural products by blood-brain barrier specific permeability assay combined with DPPH recognition. J. Pharm. Biomed. Anal. 2018, 151, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Li, Y.; Liang, X.; Yang, L.; Su, M.; Lai, K.P. Network pharmacology and bioinformatics analyses identify intersection genes of niacin and COVID-19 as potential therapeutic targets. Brief. Bioinform. 2021, 22, 1279–1290. [Google Scholar] [CrossRef]
- Zhu, J.F.; Yi, X.J.; Huang, P.; Chen, S.Q.; Wu, Y.J. Drug-protein binding of Danhong injection and the potential influence of drug combination with aspirin: Insight by ultrafiltration LC-MS and molecular modeling. J. Pharm. Biomed. Anal. 2017, 134, 100–107. [Google Scholar] [CrossRef]





| Plant Part | Yield | Total Phenols | Total Flavonoids |
|---|---|---|---|
| % | μg GA/mg Dry Extract | μg QE/mg Dry Extract | |
| Whole plants | 21.39 ± 1.05 | 20.32 ± 1.27 | 7.96 ± 0.87 |
| Roots | 17.99 ± 0.22 | 20.80 ± 0.35 b | 3.29 ± 0.19 d |
| Stems | 39.79 ± 4.93 | 14.49 ± 0.34 | 2.51 ± 0.09 |
| Leaves | 26.19 ± 2.39 | 22.24 ± 0.82 a,b | 12.87 ± 0.40 b,c |
| No. | tR (min) | Identification | Formula | Selected Ion | Measured m/z | Error (ppm) | MS2 |
|---|---|---|---|---|---|---|---|
| C1 | 16.188 | Quercetin 3-O-glucosyl-(1→2)-rhamnoside-7-O-glucoside | C33H40O21 | [M + H]+ | 773.2142 | 0.9 | 611.1621, 465.1041, 303.0500 |
| [M − H]− | 771.1994 | 0.6 | 609.1544, 462.0839, 301.0360, 300.0283, 299.0198 | ||||
| C2 | 16.489 | Quercetin 3-O-rhamnoside-7-O-glucoside | C27H30O16 | [M + H]+ | 611.1606 | −0.1 | 465.1004, 303.0486 |
| [M − H]− | 609.1461 | −0.7 | 463.0943, 462.0843, 447.0981, 301.0364, 300.0283, 299.0203 | ||||
| C3 | 16.925 | Kaempferol 3-O-glucosyl-(1→2)-rhamnoside-7-O-glucosyl-(1→2)-glucoside | C39H50O25 | [M + H]+ | 919.2735 | 2.3 | 757.2162, 611.1598, 449.1068, 433.1130, 287.0541 |
| [M − H]− | 917.2578 | 1.0 | 593.1622 | ||||
| C4 | 18.026 | Kaempferol 3-O-glucosyl-(1→2)-rhamnoside-7-O-glucoside | C33H40O20 | [M + H]+ | 757.2192 | 0.8 | 595.1667, 449.1080, 287.0545 |
| [M − H]− | 755.2044 | 0.5 | 593.1579, 446.0886, 285.0401, 284.0327, 283.0251 | ||||
| C5 | 22.354 | Kaempferol 3-O-(2,3-di-O-glucosyl)-rhamnoside-7-O-rhamnoside | C39H50O24 | [M + H]+ | 903.2776 | 1.2 | 741.2276, 595.1672, 433.1132, 287.0551 |
| [M − H]− | 901.2629 | 1.1 | 755.2154, 284.0326 | ||||
| C6 | 23.036 | Kaempferol 3-O-glucosyl-(1→2)-rhamnoside-7-O-rhamnoside | C33H40O19 | [M + H]+ | 741.2251 | 1.9 | 433.1141, 287.0552 |
| [M − H]− | 739.2095 | 0.5 | 593.1583, 430.0932, 413.0913, 285.0401, 284.0327, 283.0248 | ||||
| C7 | 24.855 | Kaempferol 3-O-[glucosyl-(1→4)]-[6-O-[4-hydroxy-(E)-cinnamoyl]-glucosyl-(1→3)]-6-O-[4-hydroxy-(E)-cinnamoyl]-glucosyl-(1→2)-rhamnoside | C57H62O29 | [M + H]+ | 1211.3524 | 6.1 | 903.2617, 763.2479, 595.1625, 455.1514, 449.1094, 419.1353, 309.0975, 287.0550 |
| [M − H]− | 1209.3324 | 1.7 | 1047.3040, 901.2650 |
| No. | PubChem CID | Binding Energy (kcal/mol) |
|---|---|---|
| C1 | 74978226 | −3.68 |
| C2 | 14484601 | −6.60 |
| C3 | 78100944 | −2.33 |
| C4 | 74978107 | −4.96 |
| C5 | 78100943 | −1.86 |
| C6 | 162950015 | −5.33 |
| C7 | 78100892 | −1.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Ding, H.; Zhong, L.; Xin, W.; Yi, X.; Fang, L. Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum. Molecules 2022, 27, 5698. https://doi.org/10.3390/molecules27175698
Zhu J, Ding H, Zhong L, Xin W, Yi X, Fang L. Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum. Molecules. 2022; 27(17):5698. https://doi.org/10.3390/molecules27175698
Chicago/Turabian StyleZhu, Junfeng, Haiying Ding, Like Zhong, Wenxiu Xin, Xiaojiao Yi, and Luo Fang. 2022. "Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum" Molecules 27, no. 17: 5698. https://doi.org/10.3390/molecules27175698
APA StyleZhu, J., Ding, H., Zhong, L., Xin, W., Yi, X., & Fang, L. (2022). Spectrum-Effect Relationship-Based Strategy Combined with Molecular Docking to Explore Bioactive Flavonoids from Sceptridium ternatum. Molecules, 27(17), 5698. https://doi.org/10.3390/molecules27175698