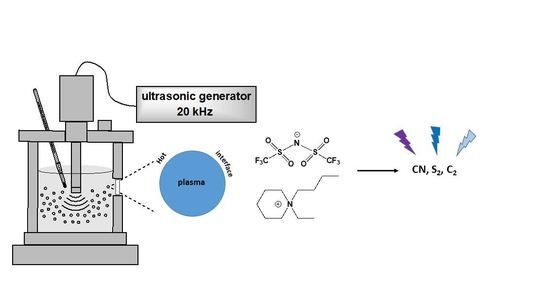
Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Follow-Up of Temperature and Estimation of Viscosity
3.2. Time Evolution of the SL Spectra of the Water-Saturated IL with 15 s Acquisition Time
3.3. Time Evolution of the SL Spectra of a Very Dry IL with 5 s Acquisition Time
3.4. Comparison of Very Dry and Water-Saturated ILs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Hagiwara, R.; Ito, Y. Room temperature ionic liquids of alkylimidazolium cations and fluoroanions. J. Fluor. Chem. 2000, 105, 221–227. [Google Scholar] [CrossRef]
- Meine, N.; Benedito, F.; Rinaldi, R. Thermal stability of ionic liquids assessed by potentiometric titration. Green Chem. 2010, 12, 1711–1714. [Google Scholar] [CrossRef]
- Li, Q.B.; Jiang, J.Y.; Li, G.F.; Zhao, W.; Zhao, X.; Mu, T. The electrochemical stability of ionic liquids and deep eutectic solvents. Sci. China Chem. 2016, 59, 571–577. [Google Scholar] [CrossRef]
- Rogers, R.D.; Seddon, K.R. Ionic liquids—Solvents of the future? Science 2003, 302, 792–793. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Naffrechoux, E.; Draye, M. Avoid the PCB, mistakes: A more sustainable future for ionic liquids. J. Hazard. Mater. 2017, 324, 773–780. [Google Scholar] [CrossRef] [PubMed]
- Chatel, G.; Goux-Henry, C.; Mirabaud, A.; Rossi, T.; Kardos, N.; Andrioletti, B.; Draye, M. H2O2/NaHCO3-mediated enantioselective epoxidation of olefins in NTf2-based ionic liquids and under ultrasound. J. Catal. 2012, 291, 127–132. [Google Scholar] [CrossRef]
- Chatel, G.; Pflieger, R.; Naffrechoux, E.; Nikitenko, S.I.; Suptil, J.; Goux-Henry, C.; Kardos, N.; Andrioletti, B.; Draye, M. Hydrophobic Bis(trifluoromethylsulfonyl)imide-Based Ionic Liquids Pyrolysis: Through the Window of the Ultrasonic Reactor. ACS Sustain. Chem. Eng. 2013, 1, 137–143. [Google Scholar] [CrossRef]
- Oxley, J.D.; Prozorov, T.; Suslick, K.S. Sonochemistry and sonoluminescence of room-temperature ionic liquids. J. Am. Chem. Soc. 2003, 125, 11138–11139. [Google Scholar] [CrossRef]
- Flannigan, D.J.; Hopkins, S.D.; Suslick, K.S. Sonochemistry and sonoluminescence in ionic liquids, molten salts, and concentrated electrolyte solutions. J. Organomet. Chem. 2005, 690, 3513–3517. [Google Scholar] [CrossRef]
- Pflieger, R.; Lejeune, M.; Noel, C.; Belmonte, T.; Nikitenko, S.I.; Draye, M. Diagnosing the plasma formed during acoustic cavitation in BEPip NTf2 ionic liquid. Phys. Chem. Chem. Phys. 2019, 21, 1183–1189. [Google Scholar] [CrossRef]
- Young, F.R. Sonoluminescence; CRC Press: New York, NY, USA, 2005. [Google Scholar]
- Pearse, R.W.B.; Gaydon, A.G. The Identification of Molecular Spectra, 4th ed.; Chapman and Hall: London, UK, 1976. [Google Scholar]
- Liu, F.G.; Zhong, X.W.; Xu, J.L.; Kamali, A.; Shi, Z. Temperature Dependence on Density, Viscosity, and Electrical Conductivity of Ionic Liquid 1-Ethyl-3-Methylimidazolium Fluoride. Appl. Sci. 2018, 8, 356. [Google Scholar] [CrossRef]
- Startsev, A.N. Diatomic sulfur: A mysterious molecule. J. Sulfur Chem. 2019, 40, 435–450. [Google Scholar] [CrossRef]
- Kaloidas, V.E.; Papayannakos, N.G. Hydrogen-production from the decomposition of hydrogen-sulfide—Equilibrium studies on the system H2S/H2/Si, (i = 1,…8) in the gas-phase. Int. J. Hydrogen Energy 1987, 12, 403–409. [Google Scholar] [CrossRef]
- Du, S.Y.; Germann, T.C.; Francisco, J.S.; Peterson, K.A.; Yu, H.G.; Lyson, J.R. The kinetics study of the S + S2 → S3 reaction by the chaperone mechanism. J. Chem. Phys. 2011, 134, 154508. [Google Scholar] [CrossRef] [PubMed]
- Fair, R.W.; Thrush, B.A. Mechanism of S2 chemiluminescence in reaction of hydrogen atoms with hydrogen sulphide. Trans. Faraday Soc. 1969, 65, 1208–1218. [Google Scholar] [CrossRef]
- Nicholas, J.E.; Amodio, C.A.; Baker, M.J. Kinetics and mechanism of the decomposition of H2S, CH3SH and (CH3)2S in a radio-frequency pulse discharge. J. Chem. Soc. Faraday Trans. I 1979, 75, 1868–1875. [Google Scholar] [CrossRef]
- Giggenbach, W.F. Redox processes governing the chemistry of fumarolic gas discharges from White Island, New Zealand. Appl. Geochem. 1987, 2, 143–161. [Google Scholar] [CrossRef]
- Mizutani, Y.; Sugiura, T. Chemical equilibrium of 2H2S + SO2 = 3S + 2H2O reaction in Solfataras of Nasudake volcano. Bull. Chem. Soc. Jpn. 1966, 39, 2411–4214. [Google Scholar] [CrossRef]
- Delmelle, P.; Bernard, A.; Kusakabe, M.; Fischer, T.P.; Takano, B. Geochemistry of the magmatic-hydrothermal system of Kawah Ijen volcano, East Java, Indonesia. J. Volcanol. Geotherm. Res. 2000, 97, 31–53. [Google Scholar] [CrossRef]
- Fair, R.W.; Thrush, B.A. Reaction between hydrogen atoms and sulphur dioxide. Trans. Faraday Soc. 1969, 65, 1157–1170. [Google Scholar] [CrossRef]
- Lee, J.; Ashokkumar, M.; Kentish, S.; Grieser, F. Effect of alcohols on the initial growth of multibubble sonoluminescence. J. Phys. Chem. B 2006, 110, 17282–17285. [Google Scholar] [CrossRef]
- Lide, D.R. Handbook of Chemistry and Physics, 90th ed.; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Tzanakis, I.; Lebon, G.S.B.; Eskin, D.G.; Pericleous, K.A. Characterizing the cavitation development and acoustic spectrum in various liquids. Ultrason. Sonochem. 2017, 34, 651–662. [Google Scholar] [CrossRef]
- Qin, D.; Zou, Q.Q.; Lei, S.; Wang, W.; Li, Z. Nonlinear dynamics and acoustic emissions of interacting cavitation bubbles in viscoelastic tissues. Ultrason. Sonochem. 2021, 78, 105712. [Google Scholar] [CrossRef]
- Lu, T.; An, Y. Effect of physical parameters on shape instability of sonoluminescing bubbles. Chin. Phys. Lett. 2006, 23, 1019–1022. [Google Scholar]
- Zilonova, E.; Solovchuk, M.; Sheu, T.W.H. Dynamics of bubble-bubble interactions experiencing viscoelastic drag. Phys. Rev. E 2019, 99, 023109. [Google Scholar] [CrossRef] [PubMed]
- Popinet, S.; Zaleski, S. Bubble collapse near a solid boundary: A numerical study of the influence of viscosity. J. Fluid Mech. 2002, 464, 137–163. [Google Scholar] [CrossRef]
- Znidarcic, A.; Mettin, R.; Cairos, C.; Dular, M. Attached cavitation at a small diameter ultrasonic horn tip. Phys. Fluids 2014, 26, 023304. [Google Scholar] [CrossRef]
- Thiemann, A.; Holsteyns, F.; Cairos, C.; Mettin, R. Sonoluminescence and dynamics of cavitation bubble populations in sulfuric acid. Ultrason. Sonochem. 2017, 34, 663–676. [Google Scholar] [CrossRef]
- Aghelmaleki, A.; Lesnik, S.; Afarideh, H.; Brenner, G.; Mettin, R. Acoustic cavitation bubble structures and dynamics in dependence of liquid viscosity. In Proceedings of the ESS-AOSS-JSS Joint Meeting, Online, 8–10 November 2021. [Google Scholar]
- Xu, H.X.; Eddingsaas, N.C.; Suslick, K.S. Spatial Separation of Cavitating Bubble Populations: The Nanodroplet Injection Model. J. Am. Chem. Soc. 2009, 131, 6060–6061. [Google Scholar] [CrossRef]
- Ban, M.; Choi, P.K. Multibubble sonoluminescence and bubble dynamics in glycerol/water mixture system. Jpn. J. Appl. Phys. 2020, 59. [Google Scholar] [CrossRef]
- Choi, P.K.; Sawada, Y.; Takeuchi, Y. Multibubble sonoluminescence pulses from Na atoms in viscous liquid. J. Acoust. Soc. Am. 2012, 131, EL413–EL419. [Google Scholar] [CrossRef] [PubMed]
- Del Sesto, R.E.; McCleskey, T.M.; Macomber, C.; Ott, C.K.; Koppisch, T.A.; Baker, A.G.; Burell, K.A. Limited thermal stability of imidazolium and pyrrolidinium ionic liquids. Thermochim. Acta 2009, 491, 118–120. [Google Scholar] [CrossRef]
- Salinas, V.; Vargas, Y.; Louisnard, O.; Gaete, L. Influence of the liquid viscosity on the formation of bubble structures in a 20 kHz field. Ultrason. Sonochem. 2015, 22, 227–234. [Google Scholar] [CrossRef] [Green Version]
- Poleski, M.; Luczak, J.; Aranowski, R.; Jungnickel, C. Wetting of surfaces with ionic liquids. Physicochem. Probl. Miner. Process. 2013, 49, 277–286. [Google Scholar]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pflieger, R.; Lejeune, M.; Draye, M. Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar. Molecules 2022, 27, 6050. https://doi.org/10.3390/molecules27186050
Pflieger R, Lejeune M, Draye M. Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar. Molecules. 2022; 27(18):6050. https://doi.org/10.3390/molecules27186050
Chicago/Turabian StylePflieger, Rachel, Manuel Lejeune, and Micheline Draye. 2022. "Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar" Molecules 27, no. 18: 6050. https://doi.org/10.3390/molecules27186050
APA StylePflieger, R., Lejeune, M., & Draye, M. (2022). Sonoluminescence Spectra in the First Tens of Seconds of Sonolysis of [BEPip][NTf2], at 20 kHz under Ar. Molecules, 27(18), 6050. https://doi.org/10.3390/molecules27186050