Pollution, Exposure and Risk of Biogenic Amines in Canned Sea Fish: Classification of Analytical Methods Based on Carbon Spheres QuEChERS Extraction Combined with HPLC
Abstract
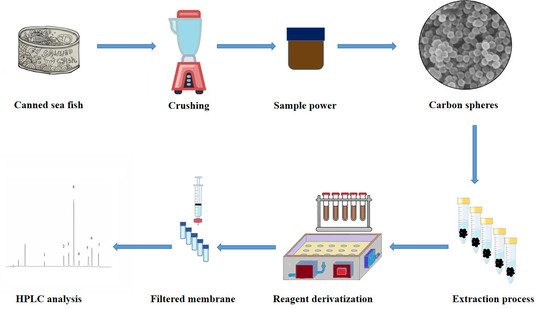
:1. Introduction
2. Results and Discussion
2.1. Morphological Characterization of Carbon Spheres
2.2. Optimization of Sample Preparation
2.2.1. Sample Extraction
2.2.2. Sample Cleanup
2.3. Method Validation
2.3.1. Linearity and Sensitivity
2.3.2. Precision and Recovery
2.4. Health Risk Assessment Model
2.4.1. Assessment of Dietary Exposure
2.4.2. Food Safety Index Evaluation
2.5. Samples Pollution Levels
2.6. Samples Risk Assessments
2.6.1. Dietary Exposure Assessment
2.6.2. Food Safety Index Evaluation
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Sample
3.3. Preparation of Standard Solutions
3.4. Preparation of Phenolic Resin Based Carbon Spheres (PFC/CS)
3.5. Sample Preparation and Pre-Column Derivatization
3.5.1. Extraction
3.5.2. Purification
3.5.3. Derivatization
3.6. Instrumentation and Analytical Conditions
3.7. Method Validation
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kubicskó, K.; Farkas, Ö. Quantum chemical (QM:MM) investigation of the mechanism of enzymatic reaction of tryptamine and N,N-dimethyltryptamine with monoamine oxidase A. Org. Biomol. Chem. 2020, 18, 9660–9674. [Google Scholar] [CrossRef] [PubMed]
- Topuz, O.K.; Yatmaz, H.A.; Alp, A.C.; Kaya, A.; Yerlikaya, P. Biogenic amine formation in fish roe in under the effect of drying methods and coating materials. J. Food Process. Preserv. 2020, 45, e15052. [Google Scholar] [CrossRef]
- Houicher, A.; Bensid, A.; Regenstein, J.M.; Özogul, F. Control of biogenic amine production and bacterial growth in fish and seafood products using phytochemicals as biopreservatives: A review. Food Biosci. 2020, 39, 100807. [Google Scholar] [CrossRef]
- Ezzat, M.; Zare, D.; Karim, R.; Ghazali, H. Trans- and cis-urocanic acid, biogenic amine and amino acid contents in ikan pekasam (fermented fish) produced from Javanese carp (Puntius gonionotus) and black tilapia (Oreochromis mossambicus). Food Chem. 2015, 172, 893–899. [Google Scholar] [CrossRef]
- Hassan, A.; Shater, A.E.; Waly, A. Histamine as biogenic amine residue in imported frozen fish. Benha Vet. Med. J. 2017, 32, 75–78. [Google Scholar] [CrossRef]
- Hwang, C.-C.; Lin, C.-M.; Huang, C.-Y.; Huang, Y.-L.; Kang, F.-C.; Hwang, D.-F.; Tsai, Y.-H. Chemical characterisation, biogenic amines contents, and identification of fish species in cod and escolar steaks, and salted escolar roe products. Food Control 2012, 25, 415–420. [Google Scholar] [CrossRef]
- Al Bulushi, I.; Poole, S.; Deeth, H.C.; Dykes, G. Biogenic Amines in Fish: Roles in Intoxication, Spoilage, and Nitrosamine Formation—A Review. Crit. Rev. Food Sci. Nutr. 2009, 49, 369–377. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.; Gopal, T.K.S. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- Pawar, R.S.; Grundel, E.; Fardin-Kia, A.R.; Rader, J.I. Determination of selected biogenic amines in Acacia rigidula plant materials and dietary supplements using LC–MS/MS methods. J. Pharm. Biomed. Anal. 2014, 88, 457–466. [Google Scholar] [CrossRef]
- Filip, N.; Mircea, C.; Iancu, C.; Hăncianu, M.; Cojocaru, E.; Stoica, B.; Filip, C. Determination of some biogenic amines in rat plasma using high performance liquid chromatography tandem mass spectrometry (HPLC/MS) method. Farmacia 2018, 66, 548–552. [Google Scholar] [CrossRef]
- Du, L.; Lao, Y.; Sasaki, Y.; Lyu, X.; Gao, P.; Wu, S.; Minami, T.; Liu, Y. Freshness monitoring of raw fish by detecting biogenic amines using a gold nanoparticle-based colorimetric sensor array. RSC Adv. 2022, 12, 6803–6810. [Google Scholar] [CrossRef] [PubMed]
- Weremfo, A.; Eduafo, M.K.; Gyimah, H.A.; Abassah-Oppong, S. Monitoring the Levels of Biogenic Amines in Canned Fish Products Marketed in Ghana. J. Food Qual. 2020, 2020, 2684235. [Google Scholar] [CrossRef]
- Kim, N.H.; Park, Y.; Jeong, E.S.; Kim, C.-S.; Jeoung, M.K.; Kim, K.S.; Hong, S.-H.; Son, J.-K.; Hong, J.T.; Park, I.-Y.; et al. A liquid chromatographic method for the determination of histamine in immunoglobulin preparation using solid phase extraction and pre-column derivatization. Arch. Pharmacal Res. 2007, 30, 1350–1357. [Google Scholar] [CrossRef]
- Salazar, M.T.; Smith, T.K.; Harris, A. High-Performance Liquid Chromatographic Method for Determination of Biogenic Amines in Feedstuffs, Complete Feeds, and Animal Tissues. J. Agric. Food Chem. 2000, 48, 1708–1712. [Google Scholar] [CrossRef] [PubMed]
- Brückner, H.; Flassig, S.; Kirschbaum, J. Determination of biogenic amines in infusions of tea (Camellia sinensis) by HPLC after derivatization with 9-fluorenylmethoxycarbonyl chloride (Fmoc-Cl). Amino Acids 2012, 42, 877–885. [Google Scholar] [CrossRef]
- Zhang, L.-Y.; Sun, M.-X. Determination of histamine and histidine by capillary zone electrophoresis with pre-column naphthalene-2,3-dicarboxaldehyde derivatization and fluorescence detection. J. Chromatogr. A 2004, 1040, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Abid, F.M.; Salman, J.M. Liquid Chromatographic Method for Determination of Biogenic Amines in Imported Fish and Meat Products. Asian J. Chem. 2012, 24, 5763–5765. [Google Scholar] [CrossRef]
- Kounnoun, A.; EL Maadoudi, M.; Cacciola, F.; Mondello, L.; Bougtaib, H.; Alahlah, N.; Amajoud, N.; EL Baaboua, A.; Louajri, A. Development and Validation of a High-Performance Liquid Chromatography Method for the Determination of Histamine in Fish Samples Using Fluorescence Detection with Pre-column Derivatization. Chromatographia 2020, 83, 893–901. [Google Scholar] [CrossRef]
- Park, J.S.; Lee, C.H.; Kwon, E.Y.; Lee, H.J.; Kim, J.Y.; Kim, S.H. Monitoring the contents of biogenic amines in fish and fish products consumed in Korea. Food Control 2010, 21, 1219–1226. [Google Scholar] [CrossRef]
- Chen, R.; Deng, Y.; Yang, L.; Wang, J.; Xu, F. Determination of Histamine by High-Performance Liquid Chromatography After Precolumn Derivatization with o-Phthalaldehyde-Sulfite. J. Chromatogr. Sci. 2015, 54, 547–553. [Google Scholar] [CrossRef] [Green Version]
- Muscarella, M.; Magro, S.L.; Campaniello, M.; Armentano, A.; Stacchini, P. Survey of histamine levels in fresh fish and fish products collected in Puglia (Italy) by ELISA and HPLC with fluorimetric detection. Food Control 2013, 31, 211–217. [Google Scholar] [CrossRef]
- Evangelista, W.P.; Silva, T.M.; Guidi, L.R.; Tette, P.A.; Byrro, R.M.; Santiago-Silva, P.; Fernandes, C.; Gloria, M.B.A. Quality assurance of histamine analysis in fresh and canned fish. Food Chem. 2016, 211, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Magro, S.L.; Summa, S.; Iammarino, M.; D’Antini, P.; Marchesani, G.; Chiaravalle, A.; Muscarella, M. A 5-Years (2015–2019) Control Activity of an EU Laboratory: Contamination of Histamine in Fish Products and Exposure Assessment. Appl. Sci. 2020, 10, 8693. [Google Scholar] [CrossRef]
- Afé, O.H.I.; Saegerman, C.; Kpoclou, Y.E.; Douny, C.; Igout, A.; Mahillon, J.; Anihouvi, V.B.; Hounhouigan, D.J.; Scippo, M.-L. Contamination of smoked fish and smoked-dried fish with polycyclic aromatic hydrocarbons and biogenic amines and risk assessment for the Beninese consumers. Food Control 2021, 126, 108089. [Google Scholar] [CrossRef]
- Koral, S.; Tufan, B.; Ščavničar, A.; Kočar, D.; Pompe, M.; Köse, S. Investigation of the contents of biogenic amines and some food safety parameters of various commercially salted fish products. Food Control 2013, 32, 597–606. [Google Scholar] [CrossRef]
- European Food Safety Authority. Scientific Opinion on Risk Based Control of Biogenic Amine Formation in Fermented Foods [EB/OL]. 2011. Available online: http://www.efsa.europa.eu/ (accessed on 11 October 2011).
- Guo, X.; Zhang, W.; Gu, J.; Chen, F.; Yang, Q. The determination of the level, source, and risk of polycyclic aromatic hydrocarbon content in traditional Chinese medicines using a QuEChERS based extraction and HPLC-UV-FLD analysis. J. Liq. Chromatogr. Relat. Technol. 2021, 44, 210–219. [Google Scholar] [CrossRef]
- Inagaki, M.; Sunahara, M. Gas adsorption-desorption behavior of activated carbon spheres derived from phenol resin. Carbon 1998, 36, 1875. [Google Scholar] [CrossRef]
- Inagaki, M.; Vignal, V.; Konno, H.; Morawski, A.W. Effects of carbonization atmosphere and subsequent oxidation on pore structure of carbon spheres observed by scanning tunneling microscopy. J. Mater. Res. 1999, 14, 3152–3157. [Google Scholar] [CrossRef]







| NO. | BAs | Abb | Time (min) | Curve | R2 | LOD (μg/kg) | LOQ (μg/kg) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Tryptamine | Try | 13.114 | y = 39.488x + 18.55 | 0.9997 | 10.8 | 36 | 95.6 | 1.9 |
| 2 | Putrescine | Put | 19.376 | y = 6.659x + 18.78 | 0.9996 | 10.8 | 36 | 92.3 | 3.7 |
| 3 | Cadaverine | Cad | 20.991 | y = 33.069x + 55.44 | 0.9998 | 7.2 | 24 | 93.1 | 4.8 |
| 4 | Histamine | His | 21.882 | y = 133.1x + 18.49 | 0.9999 | 7.2 | 24 | 97.7 | 2.3 |
| 5 | Tyramine | Tyr | 27.353 | y = 96.95x + 11.41 | 0.9998 | 10.8 | 36 | 94.2 | 3.2 |
| 6 | Spermidine | Spd | 28.494 | y = 89.147x + 29.56 | 1 | 7.2 | 24 | 92.6 | 4.1 |
| 7 | Spermine | Spm | 32.142 | y = 94.954x + 10.05 | 1 | 7.2 | 24 | 93.5 | 2.6 |
| Type | Tryptamine | Putrescine | Cadaverine | Histamine | Tyramine | Spermidine | Spermine | ∑7 BAs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | Mean | Range | |
| Tunas | ND | ND | 29.34 | ND~142.63 | 7.51 | ND~118.36 | 19.59 | ND~167.02 | 6.98 | ND~75.97 | 1.92 | ND~19.74 | 1.46 | ND~18.77 | 66.81 | ND~231.05 |
| Sardine | 1.22 | ND~29.45 | 27.99 | ND~117.81 | 20.80 | ND~165.82 | 29.69 | ND~122.45 | 28.66 | ND~115.60 | 2.60 | ND~26.12 | 1.31 | ND~17.63 | 112.27 | ND~274.49 |
| Anchovy | 0.75 | ND~18.10 | 24.09 | ND~89.97 | 21.52 | ND~153.63 | 30.50 | ND~75.47 | 18.41 | ND~87.25 | 1.60 | ND~19.17 | 1.03 | ND~16.19 | 97.91 | ND~292.47 |
| Mackerel | ND | ND | 21.74 | ND~82.80 | 25.84 | ND~123.15 | 23.07 | ND~95.62 | 12.62 | ND~50.64 | ND | ND | 0.80 | ND~11.26 | 84.07 | ND~207.65 |
| Spanish mackerel | ND | ND | 9.76 | ND~29.10 | 5.53 | ND~33.20 | 85.44 | 29.40~282.50 | 9.25 | ND~23.70 | 10.21 | ND~46.80 | 1.59 | ND~9.52 | 121.77 | 29.40~368.50 |
| Chub mackerel | ND | ND | 31.49 | ND~104.03 | 14.16 | ND~70.80 | 17.75 | ND~59.13 | 25.70 | ND~83.00 | 5.32 | ND~26.60 | 2.71 | ND~13.53 | 97.12 | ND~235.84 |
| Saury | ND | ND | 24.85 | ND~99.40 | ND | ND | 11.58 | ND~46.30 | 6.85 | ND~27.40 | ND | ND | ND | ND | 43.27 | ND~126.80 |
| Bream | ND | ND | 9.12 | ND~27.37 | ND | ND | 42.67 | 12.00~82.36 | 20.17 | ND~41.43 | 9.14 | ND~27.41 | 4.11 | ND~12.32 | 85.21 | 61.06~123.79 |
| Average | 0.45 | ND~29.45 | 25.81 | ND~142.63 | 15.14 | ND~165.82 | 27.74 | ND~282.50 | 16.25 | ND~115.60 | 2.44 | ND~46.80 | 1.34 | ND~18.77 | 89.18 | ND~368.50 |
| Type | Tryptamine | Putrescine | Cadaverine | Histamine | Tyramine | Spermidine | Spermine | ∑7 BAs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | Dr | P50/P25/P75 | |
| Tunas | ND | ND/ND/ND | 54.76 | 21.20/ND/51.04 | 14.29 | ND/ND/ND | 59.52 | 13.60/ND/26.30 | 14.29 | ND/ND/ND | 14.29 | ND/ND/ND | 11.90 | ND/ND/ND | 83.33 | 64.97/17.96/87.23 |
| Sardine | 6.06 | ND/ND/ND | 51.52 | 11.04/ND/51.91 | 30.30 | ND/ND/31.99 | 72.73 | 23.40/ND/41.62 | 57.58 | 18.63/ND/54.17 | 15.15 | ND/ND/ND | 9.09 | ND/ND/ND | 84.85 | 86.84/41.26/195.53 |
| Anchovy | 4.17 | ND/ND/ND | 50.00 | 6.06/ND/48.71 | 29.17 | ND/ND/22.77 | 87.50 | 19.14/13.80/60.86 | 45.83 | 12.32/ND/33.93 | 12.50 | ND/ND/ND | 8.33 | ND/ND/ND | 87.50 | 93.72/15.80/141.29 |
| Mackerel | ND | ND/ND/ND | 57.14 | 17.51/ND/34.39 | 35.71 | ND/ND/57.07 | 57.14 | 12.31/ND/43.47 | 42.86 | ND/ND/25.58 | ND | ND/ND/ND | 7.14 | ND/ND/ND | 85.71 | 75.50/26.66/141.40 |
| Spanish mackerel | ND | ND/ND/ND | 50.00 | 6.39/ND/16.66 | 16.67 | ND/ND/ND | 100 | 51.75/29.40/67.80 | 50.00 | 6.83/ND/18.11 | 33.33 | ND/ND/14.49 | 16.67 | ND/ND/ND | 100 | 45.10/87.22/113.17 |
| Chub mackerel | ND | ND/ND/ND | 40.00 | ND/ND/53.40 | 20.00 | ND/ND/ND | 40.00 | ND/ND/29.60 | 60.00 | ND/ND/25.90 | 20.00 | ND/ND/ND | 20.00 | ND/ND/ND | 80.00 | 43.13/19.60/187.03 |
| Saury | ND | ND/ND/ND | 25.00 | ND/ND/ND | ND | ND/ND/ND | 25.00 | ND/ND/ND | 25.00 | ND/ND/ND | ND | ND/ND/ND | ND | ND/ND/46.30 | 50.00 | 23.15/ND/46.30 |
| Bream | ND | ND/ND/ND | 33.33 | ND/ND/27.37 | ND | ND/ND/ND | 100 | 33.65/11.99/82.36 | 66.67 | ND/ND/27.41 | 33.33 | ND/ND/12.32 | 33.33 | 70.77/61.06/123.79 | 100 | 58.44/33.65/123.79 |
| Average | 2.29 | ND/ND/ND | 51.15 | 11.04/ND/46.61 | 22.90 | ND/ND/ND | 69.47 | 16.72/ND/36.41 | 40.46 | ND/ND/24.50 | 12.98 | ND/ND/ND | 10.69 | ND/ND/ND | 83.97 | 74.68/21.40/121.85 |
| Type | Age | Gender | Exposure (μg·kg−1·d−1 ) | Food Safety Index (IFSc) | ||||
|---|---|---|---|---|---|---|---|---|
| Nationwide | City | Countryside | Nationwide | City | Countryside | |||
| Infant | 2~3 | Male | 209.52 | 214.99 | 163.42 | 0.0195 | 0.0200 | 0.0152 |
| Infant | 2~3 | Female | 120.97 | 144.30 | 83.75 | 0.0113 | 0.0135 | 0.0078 |
| Children | 4~10 | Male | 115.43 | 116.31 | 99.20 | 0.0108 | 0.0108 | 0.0092 |
| Children | 4~10 | Female | 130.43 | 133.14 | 90.72 | 0.0122 | 0.0124 | 0.0085 |
| Immaturity | 11~17 | Male | 93.32 | 98.51 | 60.76 | 0.0087 | 0.0092 | 0.0057 |
| Immaturity | 11~17 | Female | 70.19 | 76.44 | 49.81 | 0.0065 | 0.0071 | 0.0046 |
| Youth | 18~44 | Male | 67.03 | 69.08 | 59.33 | 0.0062 | 0.0064 | 0.0055 |
| Youth | 18~44 | Female | 72.23 | 73.86 | 67.24 | 0.0067 | 0.0069 | 0.0063 |
| Middle age | 45~59 | Male | 69.72 | 69.66 | 66.43 | 0.0065 | 0.0065 | 0.0062 |
| Middle age | 45~59 | Female | 74.09 | 73.56 | 73.98 | 0.0069 | 0.0069 | 0.0069 |
| Old age | >60 | Male | 83.04 | 73.87 | 99.86 | 0.0077 | 0.0069 | 0.0093 |
| Old age | > 60 | Female | 90.45 | 86.49 | 96.47 | 0.0084 | 0.0081 | 0.0090 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, X.; Dai, Z.; Zhang, W. Pollution, Exposure and Risk of Biogenic Amines in Canned Sea Fish: Classification of Analytical Methods Based on Carbon Spheres QuEChERS Extraction Combined with HPLC. Molecules 2022, 27, 6243. https://doi.org/10.3390/molecules27196243
Guo X, Dai Z, Zhang W. Pollution, Exposure and Risk of Biogenic Amines in Canned Sea Fish: Classification of Analytical Methods Based on Carbon Spheres QuEChERS Extraction Combined with HPLC. Molecules. 2022; 27(19):6243. https://doi.org/10.3390/molecules27196243
Chicago/Turabian StyleGuo, Xinying, Zhiying Dai, and Weibing Zhang. 2022. "Pollution, Exposure and Risk of Biogenic Amines in Canned Sea Fish: Classification of Analytical Methods Based on Carbon Spheres QuEChERS Extraction Combined with HPLC" Molecules 27, no. 19: 6243. https://doi.org/10.3390/molecules27196243
APA StyleGuo, X., Dai, Z., & Zhang, W. (2022). Pollution, Exposure and Risk of Biogenic Amines in Canned Sea Fish: Classification of Analytical Methods Based on Carbon Spheres QuEChERS Extraction Combined with HPLC. Molecules, 27(19), 6243. https://doi.org/10.3390/molecules27196243