Canavalia gladiata Pod Extract Mitigates Ovalbumin-Induced Asthma Onset in Male BALB/c Mice via Suppression of MAPK
Abstract
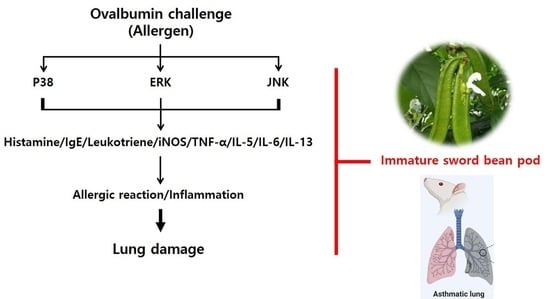
:1. Introduction
2. Results and Discussion
2.1. Free Phenolic Acid, Antioxidant Capacity, and Phytochemicals in SBP
2.2. Effects of SBP on OVA/Alum.-Induced Pulmonary Damage
2.3. Effects of SBP on OVA/Alum.-Induced Inflammation
2.4. Effects of SBP Treatment on OVA/Alum.-Induced Signaling Pathways
3. Materials and Methods
3.1. Materials and Reagents
3.2. Analysis of Free Phenolic Acid
3.3. Antioxidant Capacity and Component Assay
3.3.1. ABTS Radical Scavenging Assay
3.3.2. DPPH Radical Scavenging Assay
3.3.3. Total Polyphenol Content
3.3.4. Total Flavonoid Content
3.4. Animals and Treatment
3.5. Histological Analysis
3.6. ELISA Assay
3.7. Immunoblot Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Principe, S.; Porsbjerg, C.; Bolm Ditlev, S.; Kjærsgaard Klein, D.; Golebski, K.; Dyhre-Petersen, N.; van Dijk, Y.E.; van Bragt, J.J.; Dankelman, L.L.; Dahlen, S. Treating Severe Asthma: Targeting the IL-5 Pathway. Clin. Exp. Allergy 2021, 51, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Tiotiu, A.I.; Novakova, P.; Nedeva, D.; Chong-Neto, H.J.; Novakova, S.; Steiropoulos, P.; Kowal, K. Impact of Air Pollution on Asthma Outcomes. Int. J. Environ. Res. Public Health 2020, 17, 6212. [Google Scholar] [CrossRef]
- Chen, F.; He, D.; Yan, B. Apigenin Attenuates Allergic Responses of Ovalbumin-Induced Allergic Rhinitis through Modulation of Th1/Th2 Responses in Experimental Mice. Dose-Response 2020, 18, 1559325820904799. [Google Scholar] [CrossRef]
- Elieh, A.K.D.; Bjermer, L. Mast Cell-Mediated Orchestration of the Immune Responses in Human Allergic Asthma: Current Insights. Clin. Rev. Allergy Immunol. 2019, 56, 234–247. [Google Scholar] [CrossRef] [PubMed]
- Kandhare, A.D.; Liu, Z.; Mukherjee, A.A.; Bodhankar, S.L. Therapeutic Potential of Morin in Ovalbumin-Induced Allergic Asthma Via Modulation of SUMF2/IL-13 and BLT2/NF-kB Signaling Pathway. Curr. Mol. Pharmacol. 2019, 12, 122–138. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.S.; Singh, D.; Dash, D.; Singh, R. Intranasal Curcumin Regulates Chronic Asthma in Mice by Modulating NF-ĸB Activation and MAPK Signaling. Phytomedicine 2018, 51, 29–38. [Google Scholar] [CrossRef]
- Yuan, F.; Liu, R.; Hu, M.; Rong, X.; Bai, L.; Xu, L.; Mao, Y.; Hasimu, H.; Sun, Y.; He, J. JAX2, an Ethanol Extract of Hyssopus Cuspidatus Boriss, can Prevent Bronchial Asthma by Inhibiting MAPK/NF-κB Inflammatory Signaling. Phytomedicine 2019, 57, 305–314. [Google Scholar] [CrossRef]
- Huang, L.; Wang, M.; Yan, Y.; Gu, W.; Zhang, X.; Tan, J.; Sun, H.; Ji, W.; Chen, Z. OX40L Induces Helper T Cell Differentiation during Cell Immunity of Asthma through PI3K/AKT and P38 MAPK Signaling Pathway. J. Transl. Med. 2018, 16, 1–10. [Google Scholar] [CrossRef]
- Pelaia, C.; Vatrella, A.; Crimi, C.; Gallelli, L.; Terracciano, R.; Pelaia, G. Clinical Relevance of Understanding Mitogen-Activated Protein Kinases Involved in Asthma. Expert Rev. Respir. Med. 2020, 14, 501–510. [Google Scholar] [CrossRef]
- Bleecker, E.R.; Menzies-Gow, A.N.; Price, D.B.; Bourdin, A.; Sweet, S.; Martin, A.L.; Alacqua, M.; Tran, T.N. Systematic Literature Review of Systemic Corticosteroid use for Asthma Management. Am. J. Respir. Crit. Care Med. 2020, 201, 276–293. [Google Scholar] [CrossRef]
- Hwang, K.; Heo, W.; Hwang, H.; Han, B.K.; Song, M.C.; Kim, Y.J. Anti-Inflammatory Effect of Immature Sword Bean Pod (Canavalia Gladiata) in Lipopolysaccharide-Induced RAW264. 7 Cells. J. Med. Food 2020, 23, 1183–1191. [Google Scholar] [CrossRef] [PubMed]
- Price, D.B.; Trudo, F.; Voorham, J.; Xu, X.; Kerkhof, M.; Ling Zhi Jie, J.; Tran, T.N. Adverse Outcomes from Initiation of Systemic Corticosteroids for Asthma: Long-Term Observational Study. J. Asthma Allergy 2018, 11, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Kuralkar, P.; Kuralkar, S. Role of Herbal Products in Animal production—An Updated Review. J. Ethnopharmacol. 2021, 278, 114246. [Google Scholar] [CrossRef]
- Parasuraman, S. Herbal Drug Discovery: Challenges and Perspectives. Curr. Pharm. Pers. Med. 2018, 16, 63–68. [Google Scholar] [CrossRef]
- Zhao, J. Characterization of the Complete Chloroplast Genome of Canavalia Gladiata. Mitochondrial DNA Part. B 2021, 6, 252–253. [Google Scholar] [CrossRef]
- Lee, H.; Park, J.U.; Guo, R.H.; Kang, B.Y.; Park, I.; Kim, Y.R. Anti-Inflammatory Effects of Canavalia Gladiata in Macrophage Cells and DSS-Induced Colitis Mouse Model. Am. J. Chin. Med. 2019, 47, 1571–1588. [Google Scholar] [CrossRef]
- Hwang, H.; Hwang, Y.J.; Kim, Y.J.; Kim, M.; Hwang, K. Immature Sword Bean Pods (Canavalia Gladiata) Inhibit Adipogenesis in C3H10T1/2 Cells and Mice with High-Fat diet–induced Obesity. J. Chin. Med. Assoc. 2022, 85, 67–76. [Google Scholar] [CrossRef]
- Hwang, K.; Hwang, Y.J.; Hwang, H.; Lee, S.H.; Kim, Y.J. Sword Bean (Canavalia Gladiata) Pod Exerts Anti-Allergic and Anti-Inflammatory Effects through Modulation of Th1/Th2 Cell Differentiation. Nutrients 2022, 14, 2853. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, H.; Ma, C.; Lv, L.; Feng, J.; Han, S. Gallic Acid Attenuates Allergic Airway Inflammation Via Suppressed interleukin-33 and Group 2 Innate Lymphoid Cells in ovalbumin-induced Asthma in Mice. Int. Forum Allergy Rhinol. 2018, 8, 1284–1290. [Google Scholar] [CrossRef]
- Bai, F.; Fang, L.; Hu, H.; Yang, Y.; Feng, X.; Sun, D. Vanillic Acid Mitigates the Ovalbumin (OVA)-Induced Asthma in Rat Model through Prevention of Airway Inflammation. Biosci. Biotechnol. Biochem. 2019, 83, 531–537. [Google Scholar] [CrossRef]
- Lee, C.; Wang, C.; Huang, H.; Lin, C.; Leu, S.; Lee, Y. Ferulic Acid Induces Th1 Responses by Modulating the Function of Dendritic Cells and Ameliorates Th2-Mediated Allergic Airway Inflammation in Mice. Evid. Based Complement. Altern. Med. 2015, 2015, 1–16. [Google Scholar]
- Bai, J.; Zhang, Y.; Tang, C.; Hou, Y.; Ai, X.; Chen, X.; Zhang, Y.; Wang, X.; Meng, X. Gallic Acid: Pharmacological Activities and Molecular Mechanisms Involved in Inflammation-Related Diseases. Biomed. Pharmacother. 2021, 133, 110985. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, J.; Liu, Y.; Ge, A.; Gu, H.; Zha, W.; Zeng, X.; Huang, M. Caffeic Acid Phenethyl Ester Alleviates Asthma by Regulating the Airway Microenvironment Via the ROS-Responsive MAPK/Akt Pathway. Free Radic. Biol. Med. 2016, 101, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, S.; Liu, X.; Xu, J.; Jiang, A.; Zhang, Y.; Liu, Z.; Wang, J.; Zhou, E.; Wei, Z. Alpinetin Prevents Inflammatory Responses in OVA-Induced Allergic Asthma through Modulating PI3K/AKT/NF-κB and HO-1 Signaling Pathways in Mice. Int. Immunopharmacol. 2020, 89, 107073. [Google Scholar] [CrossRef]
- Wu, W.; Li, Y.; Jiao, Z.; Zhang, L.; Wang, X.; Qin, R. Phyllanthin and Hypophyllanthin from Phyllanthus Amarus Ameliorates Immune-Inflammatory Response in Ovalbumin-Induced Asthma: Role of IgE, Nrf2, iNOs, TNF-α, and IL’s. Immunopharmacol. Immunotoxicol. 2019, 41, 55–67. [Google Scholar] [CrossRef]
- Shou, Q.; Lang, J.; Jin, L.; Fang, M.; Cao, B.; Cai, Y.; Ni, Z.; Qiu, F.; Li, C.; Cao, G. Total Glucosides of Peony Improve Ovalbumin-Induced Allergic Asthma by Inhibiting Mast Cell Degranulation. J. Ethnopharmacol. 2019, 244, 112136. [Google Scholar] [CrossRef]
- Yao, Y.; Miao, X.; Wang, L.; Jiang, Z.; Li, L.; Jiang, P.; Wang, Y.; Jin, A.; Li, N.; Wang, C. Methane Alleviates Lung Injury through the IL-10 Pathway by Increasing T Regulatory Cells in a Mouse Asthma Model. J. Immunol. Res. 2022, 2022, 6008376. [Google Scholar] [CrossRef]
- Boonpiyathad, T.; Sözener, Z.C.; Satitsuksanoa, P.; Akdis, C.A. Immunologic Mechanisms in Asthma. Semin. Immunol. 2019, 46, 101333. [Google Scholar] [CrossRef]
- León, B.; Ballesteros-Tato, A. Modulating Th2 Cell Immunity for the Treatment of Asthma. Front. Immunol. 2021, 12, 637948. [Google Scholar] [CrossRef]
- Lee, I.; Yang, C. Inflammatory Signalings Involved in Airway and Pulmonary Diseases. Mediat. Inflamm. 2013, 2013, 1–12. [Google Scholar] [CrossRef]
- Kang, W.; Jia, Z.; Tang, D.; Zhang, Z.; Gao, H.; He, K.; Feng, Q. Fusobacterium Nucleatum Facilitates Apoptosis, ROS Generation, and Inflammatory Cytokine Production by Activating AKT/MAPK and NF-κB Signaling Pathways in Human Gingival Fibroblasts. Oxid. Med. Cell. Longev. 2019, 2019, 1681972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitra, S.; Ghosh, N.; Paul, P.; Banerjee, E.R. Orally Administered Fisetin Reduces the Symptoms of Acute Allergic Asthma in a Preclinical Mouse Model. Biomed. Res. Ther. 2022, 9, 4953–4970. [Google Scholar] [CrossRef]
- Pelaia, G.; Vatrella, A.; Busceti, M.T.; Gallelli, L.; Calabrese, C.; Terracciano, R.; Maselli, R. Cellular Mechanisms Underlying Eosinophilic and Neutrophilic Airway Inflammation in Asthma. Mediat. Inflamm. 2015, 2015, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Seyfizadeh, N.; Gharibi, T.; Babaloo, Z. Interleukin-13 as an Important Cytokine: A Review on its Roles in some Human Diseases. Acta Microbiol. Immunol. Hung. 2015, 62, 341–378. [Google Scholar] [CrossRef]
- Zhao, J.; O’Donnell, V.B.; Balzar, S.; St. Croix, C.M.; Trudeau, J.B.; Wenzel, S.E. 15-Lipoxygenase 1 Interacts with Phosphatidylethanolamine-Binding Protein to Regulate MAPK Signaling in Human Airway Epithelial Cells. Proc. Natl. Acad. Sci. USA 2011, 108, 14246–14251. [Google Scholar] [CrossRef]
- Shalaby, E.A.; Shanab, S.M. Comparison of DPPH and ABTS assays for determining antioxidant potential of water and methanol extracts of Spirulina platensis. Indian J. Geo. Mar. Sci. 2013, 42, 556–564. [Google Scholar]
- Singh, R.; Verma, P.; Singh, G. Total phenolic, flavonoids and tannin contents in different extracts of Artemisia absinthium. J. Intercult. Ethnopharmacol. 2012, 1, 101–104. [Google Scholar] [CrossRef]
- Huang, W.; Heo, W.; Jeong, I.; Kim, M.; Han, B.; Shin, E.; Kim, Y. Ameliorative Effect of Citrus Junos Tanaka Waste (by-Product) Water Extract on Particulate Matter 10-Induced Lung Damage. Nutrients 2022, 14, 2270. [Google Scholar] [CrossRef]





| ABTS Assay | DPPH Assay | Total Polyphenol Content | Total Flavonoid Content |
|---|---|---|---|
| mg VCE/g sample | mg GAE/g sample | mg RE/g sample | |
| 31.61 ± 0.19 | 20.61 ± 1.06 | 16.52 ± 0.21 | 6.82 ± 0.13 |
| Gallic Acid | p-Hydroxybenzoic Acid | Vanillic Acid | Caffeic Acid | Ferulic Acid |
|---|---|---|---|---|
| µg/g sample (retention time (min)) | ||||
| 114.03 ± 0.83 (5.9) | 15.90 ± 0.14 (15.7) | 15.16 ± 0.04 (19.5) | 21.29 ± 0.11 (20.5) | 24.63 ± 0.11 (30.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, W.Y.; Lee, S.H.; Oh, S.J.; Yoon, H.; Pan, J.H.; Jeong, I.; Kim, M.J.; Han, B.K.; Kim, J.K.; Shin, E.-C.; et al. Canavalia gladiata Pod Extract Mitigates Ovalbumin-Induced Asthma Onset in Male BALB/c Mice via Suppression of MAPK. Molecules 2022, 27, 6317. https://doi.org/10.3390/molecules27196317
Huang WY, Lee SH, Oh SJ, Yoon H, Pan JH, Jeong I, Kim MJ, Han BK, Kim JK, Shin E-C, et al. Canavalia gladiata Pod Extract Mitigates Ovalbumin-Induced Asthma Onset in Male BALB/c Mice via Suppression of MAPK. Molecules. 2022; 27(19):6317. https://doi.org/10.3390/molecules27196317
Chicago/Turabian StyleHuang, Wen Yan, Sang Hoon Lee, Seong Ju Oh, Hyeock Yoon, Jeong Hoon Pan, Inhye Jeong, Mi Jeong Kim, Bok Kyung Han, Jae Kyeom Kim, Eui-Cheol Shin, and et al. 2022. "Canavalia gladiata Pod Extract Mitigates Ovalbumin-Induced Asthma Onset in Male BALB/c Mice via Suppression of MAPK" Molecules 27, no. 19: 6317. https://doi.org/10.3390/molecules27196317
APA StyleHuang, W. Y., Lee, S. H., Oh, S. J., Yoon, H., Pan, J. H., Jeong, I., Kim, M. J., Han, B. K., Kim, J. K., Shin, E. -C., & Kim, Y. J. (2022). Canavalia gladiata Pod Extract Mitigates Ovalbumin-Induced Asthma Onset in Male BALB/c Mice via Suppression of MAPK. Molecules, 27(19), 6317. https://doi.org/10.3390/molecules27196317