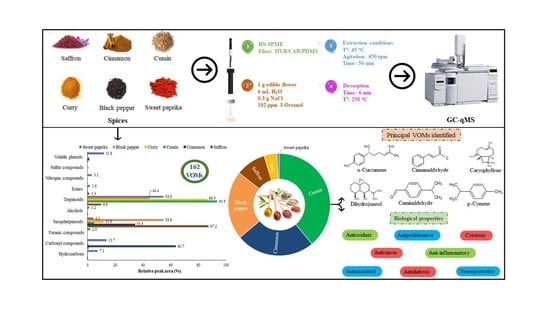
Spices Volatilomic Fingerprinting—A Comprehensive Approach to Explore Its Authentication and Bioactive Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Volatomic Fingerprinting from Spices
2.2. Saffron
2.3. Cinnamon
2.4. Cumin
2.5. Curry
2.6. Black Pepper
2.7. Sweet Paprika
2.8. Bioactive Potential of VOMs Identified in the Investigated Spices
2.9. Multivariate Statistical Data Analysis. Characterization of Spices
3. Materials and Methods
3.1. Chemical and Reagents
3.2. Spice Samples
3.3. HS-SPME Procedure
3.4. Fingerprinting of Spices by GC-MS Analysis
3.5. Data Treatment and Multivariate Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Bi, X.; Lim, J.; Henry, C.J. Spices in the management of diabetes mellitus. Food Chem. 2017, 217, 281–293. [Google Scholar] [CrossRef] [PubMed]
- Serafini, M.; Peluso, I. Functional foods for health: The interrelated antioxidant and anti-inflammatory role of fruits, vegetables, herbs, spices and cocoa in humans. Curr. Pharm. Des. 2016, 22, 6701–6715. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; He, X.Q.; Wu, D.T.; Li, H.B.; Feng, Y.B.; Zou, L.; Gan, R.Y. Elderberry (Sambucus nigra L.): Bioactive compounds, health functions, and applications. J. Agric. Food Chem. 2022, 70, 4202–4220. [Google Scholar] [CrossRef]
- Singh, N.; Rao, A.S.; Nandal, A.; Kumar, S.; Yadav, S.S.; Ganaie, S.A.; Narasimhan, B. Phytochemical and pharmacological review of Cinnamomum verum J. Presl-a versatile spice used in food and nutrition. Food Chem. 2021, 338, 127773. [Google Scholar] [CrossRef]
- Leja, K.B.; Czaczyk, K. The industrial potential of herbs and spices? A mini review. Acta Sci. Pol. Technol. Aliment. 2016, 15, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Molina, R.V.; Renau-Morata, B.; Nebauer, S.G.; Candido, V. Crocus sativus L. ecotypes from Mediterranean countries: Phenological, morpho-productive, qualitative and genetic traits. Agronomy 2021, 11, 551. [Google Scholar] [CrossRef]
- Mentis, A.A.; Dalamaga, M.; Lu, C.; Polissiou, M.G. Saffron for ‘toning down’ COVID-19-related cytokine storm: Hype or hope? A mini-review of current evidence. Metab. Open 2021, 11, 100111. [Google Scholar] [CrossRef]
- Cardone, L.; Castronuovo, D.; Perniola, M.; Cicco, N.; Candido, V. Saffron (Crocus sativus L.), the king of spices: An overview. Sci. Hortic. 2020, 272, 109560. [Google Scholar] [CrossRef]
- Isaac-Renton, M.; Li, M.K.; Parsons, L.M. Cinnamon spice and everything not nice: Many features of intraoral allergy to cinnamic aldehyde. Dermatitis 2015, 26, 116–121. [Google Scholar] [CrossRef]
- Singh, N.; Yadav, S.S.; Kumar, S.; Narashiman, B. A review on traditional uses, phytochemistry, pharmacology, and clinical research of dietary spice Cuminum cyminum L. Phytother. Res. 2021, 35, 5007–5030. [Google Scholar] [CrossRef]
- Ebada, M.E. Cuminaldehyde: A potential drug candidate. J. Pharmacol. Clin. Res. 2017, 2, 555585. [Google Scholar] [CrossRef]
- Agarwal, U.; Pathak, D.P.; Kapoor, G.; Bhutani, R.; Roper, R.; Gupta, V.; Kant, R. Review on Cuminum cyminum–Nature’s magical seeds. J. Chem. Pharm. Res. 2017, 9, 180–187. [Google Scholar]
- Takooree, H.; Aumeeruddy, M.Z.; Rengasamy, K.R.R.; Venugopala, K.N.; Jeewon, R.; Zengin, G.; Mahomoodally, M.F. A systematic review on black pepper (Piper nigrum L.): From folk uses to pharmacological applications. Crit. Rev. Food Sci. Nutr. 2019, 59 (Suppl. 1), S210–S243. [Google Scholar] [CrossRef]
- Kammath, A.J.; Nair, B.P.S.; Nath, L.R. Curry versus cancer: Potential of some selected culinary spices against cancer with in vitro, in vivo, and human trials evidences. J. Food Biochem. 2021, 45, e13285. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, Y.; Li, Y.; Xu, D.P.; Li, S.; Li, H.B. Spices for prevention and treatment of cancers. Nutrients 2016, 8, 495. [Google Scholar] [CrossRef]
- Ponder, A.; Kulik, K.; Hallmann, E. Occurrence and determination of carotenoids and polyphenols in different paprika powders from organic and conventional production. Molecules 2021, 26, 2980. [Google Scholar] [CrossRef]
- Banihashemi, S.; Bagheri, H. A core–shell titanium dioxide–polyaniline nanocomposite for the needle-trap extraction of volatile organic compounds in urine samples. J. Sep. Sci. 2017, 40, 1985–1992. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Xu, H. Graphene/polyaniline electrodeposited needle trap device for the determination of volatile organic compounds in human exhaled breath vapor and A549 cell. RSC Adv. 2017, 7, 11959–11968. [Google Scholar] [CrossRef]
- Lo, M.; Benfodda, Z.; Bénimélis, D.; Fontaine, J.-X.; Molinié, R.; Meffre, P. Extraction and identification of volatile organic compounds emitted by fragrant flowers of three Tillandsia species by HS-SPME/GC-MS. Metabolites 2021, 11, 594. [Google Scholar] [CrossRef]
- Porto-Figueira, P.; Figueira, J.A.; Berenguer, P.; Câmara, J.S. Exploring a volatomic-based strategy for a fingerprinting approach of Vaccinium padifolium L. berries at different ripening stages. Food Chem. 2018, 245, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. Tangerines cultivated on Madeira Island—A high throughput natural source of bioactive compounds. Foods 2020, 9, 1470. [Google Scholar] [CrossRef] [PubMed]
- Figueira, J.A.; Porto-Figueira, P.; Pereira, J.A.M.; Câmara, J.S. A comprehensive methodology based on NTME/GC-MS data and chemometric tools for lemons discrimination according to geographical origin. Microchem. J. 2020, 157, 104933. [Google Scholar] [CrossRef]
- Ng, F.; Thong, A.; Basri, N.; Wu, W.; Chew, W.; Dharmawan, J. Profiling of aroma-active compounds in Ylang-Ylang essential oils by aroma extract dilution analysis (AEDA) and chemometric methods. J. Agric. Food Chem. 2022, 70, 260–266. [Google Scholar] [CrossRef]
- Liu, S.; Shan, B.; Zhou, X.; Gao, W.; Liu, Y.; Zhu, B.; Sun, L. Transcriptome and metabolomics integrated analysis reveals terpene synthesis genes controlling linalool synthesis in grape berries. J. Agric. Food Chem. 2022, 70, 9084–9094. [Google Scholar] [CrossRef] [PubMed]
- Asimi, O.A.; Sahu, N.P.; Pal, A.K. Antioxidant activity and antimicrobial property of some Indian spices. Int. J. Sci. Res. Publ. 2013, 3, 1–8. [Google Scholar]
- Cozzolino, R.; Stocchero, M.; Perestrelo, R.; Câmara, J.S. Comprehensive evaluation of the volatomic fingerprint of saffron from Campania towards its authenticity and quality. Foods 2022, 11, 366. [Google Scholar] [CrossRef]
- Sandner, D.; Krings, U.; Berger, R.G. Volatiles from Cinnamomum cassia buds. Z. Naturforsch. C. J. Biosci. 2018, 73, 67–75. [Google Scholar] [CrossRef]
- Elmassry, M.M.; Kormod, L.; Labib, R.M.; Farag, M.A. Metabolome based volatiles mapping of roasted umbelliferous fruits aroma via HS-SPME GC/MS and peroxide levels analyses. J. Chromatogr. B 2018, 1099, 117–126. [Google Scholar] [CrossRef]
- Rivera-Pérez, A.; Romero-González, R.; Garrido Frenich, A. Feasibility of applying untargeted metabolomics with GC-Orbitrap-HRMS and chemometrics for authentication of black pepper (Piper nigrum L.) and identification of geographical and processing markers. J. Agric. Food Chem. 2021, 69, 5547–5558. [Google Scholar] [CrossRef]
- Kevrešan, Z.S.; Mastilović, J.S.; Sinadinović-Fišer, S.; Hrabovski, N.C.; Radusin, T.I. Spice paprika volatiles as affected by different postharvest ripening treatments of red pepper (Capsicum annuum L.) variety aleva NK. Acta Period. Technol. 2013, 44, 75–86. [Google Scholar] [CrossRef]
- Ali, A.; Wu, H.; Ponnampalam, E.N.; Cottrell, J.J.; Dunshea, F.R.; Suleria, H.A.R. Comprehensive profiling of most widely used spices for their phenolic compounds through LC-ESI-QTOF-MS2 and their antioxidant potential. Antioxidants 2021, 10, 721. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.M.; Tavaszi-Sarosi, S. Identification and quantification of essential oil content and composition, total polyphenols and antioxidant capacity of Perilla frutescens (L.) Britt. Food Chem. 2019, 275, 730–738. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Mollica, A.; Stefanucci, A.; Aumeeruddy, M.Z.; Poorneeka, R.; Zengin, G. Volatile components, pharmacological profile, and computational studies of essential oil from Aegle marmelos (Bael) leaves: A functional approach. Ind. Crops Prod. 2018, 126, 13–21. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and onions: Their cancer prevention properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef]
- Kim, T.; Song, B.; Cho, K.S.; Lee, I.S. Therapeutic potential of volatile terpenes and terpenoids from forests for inflammatory diseases. Int. J. Mol. Sci. 2020, 21, 2187. [Google Scholar] [CrossRef]
- Nazaruk, J.; Borzym-Kluczyk, M. The role of triterpenes in the management of diabetes mellitus and its complications. Phytochem. Rev. 2015, 14, 675–690. [Google Scholar] [CrossRef]
- Yang, W.; Chen, X.; Li, Y.; Guo, S.; Wang, Z.; Yu, X. Advances in pharmacological activities of terpenoids. Nat. Prod. Commun. 2020, 15, 1–13. [Google Scholar] [CrossRef]
- Aguiar, J.; Gonçalves, J.L.; Alves, V.L.; Câmara, J.S. Relationship between volatile composition and bioactive potential of vegetables and fruits of regular consumption—An integrative approach. Molecules 2021, 26, 3653. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Quispe, C.; Kumar, M.; Akram, M.; Amin, M.; Iqbal, M.; Koirala, N.; Sytar, O.; Kregiel, D.; Nicola, S.; et al. Hyssopus essential oil: An update of its phytochemistry, biological activities, and safety profile. Oxid. Med. Cell. Longev. 2022, 13, 8442734. [Google Scholar] [CrossRef]
- Tetali, S.D. Terpenes and isoprenoids: A wealth of compounds for global use. Planta 2019, 249, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Guimarães, A.C.; Meireles, L.M.; Lemos, M.F.; Guimarães, M.C.C.; Endringer, D.C.; Fronza, M.; Scherer, R. Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules 2019, 24, 2471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ansari, I.A.; Akhtar, M.S. Current insights on the role of terpenoids as anticancer agents: A perspective on cancer prevention and treatment. In Natural Bio-active Compounds: Chemistry, Pharmacology and Health Care Practices; Springer: Singapore, 2019; pp. 53–80. ISBN 978-981-13-7205-6. [Google Scholar] [CrossRef]
- Paduch, R.; Kandefer-Szerszeń, M.; Trytek, M.; Fiedurek, J. Terpenes: Substances useful in human healthcare. Arch. Immunol. Ther. Exp. 2007, 55, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Kris-Etherton, P.M.; Hecker, K.D.; Bonanome, A.; Coval, S.M.; Binkoski, A.E.; Hilpert, K.F.; Griel, A.E.; Etherton, T.D. Bioactive compounds in foods: Their role in the prevention of cardiovascular disease and cancer. Am. J. Med. 2002, 113, 71–88. [Google Scholar] [CrossRef]
- Wang, J.; Yu, H.; Zhao, Z.; Sheng, X.; Shen, Y.; Gu, H. Natural variation of glucosinolates and their breakdown products in broccoli (Brassica oleracea var. italica) seeds. J. Agric. Food Chem. 2019, 67, 12528–12537. [Google Scholar] [CrossRef]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira wine volatile profile. A platform to establish Madeira wine aroma descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- Cui, J.; Zhai, X.; Guo, D.; Du, W.; Gao, T.; Zhou, J.; Schwab, W.G.; Song, C. Characterization of key odorants in Xinyang Maojian green tea and their changes during the manufacturing process. J. Agric. Food Chem. 2022, 70, 279–288. [Google Scholar] [CrossRef]
- An, Y.; Wen, L.; Li, W.; Zhang, X.; Hu, Y.; Xiong, S. Characterization of warmed-over flavor compounds in surimi gel made from silver carp (Hypophthalmichthys molitrix) by gas chromatography-ion mobility spectrometry, aroma extract dilution analysis, aroma recombination, and omission studies. J. Agric. Food Chem. 2022, 70, 9451–9462. [Google Scholar] [CrossRef]
- Donato, F.; Biancolillo, A.; Mazzulli, D.; Rossi, L.; D’Archivio, A.A. HS-SPME/GC–MS volatile fraction determination and chemometrics for the discrimination of typical Italian Pecorino cheeses. Microchem. J. 2021, 165, 106133. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Zhou, G.; De Lima Morais, D.A.; Chang, L.; Barrette, M.; Gauthier, C.; Jacques, P.-E.; Li, S.; Xia, J. MetaboAnalyst 5.0: Narrowing the gap between raw spectra and functional insights. Nucl. Acids Res. 2021, 49, W388–W396. [Google Scholar] [CrossRef] [PubMed]




| Peak n° | RT a (min) | KIcal b | KIlit c | Volatile Metabolites | MF d | Chem. Family | Relative Area (% RSD) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saffron | Cinnamon | Cumin | Curry | Black Pepper | Sweet Paprika | |||||||
| 1 | 8.134 | 775 | 768 | 4-Methylheptane | C8H18 | HC | - | - | - | - | - | 0.16 (16) |
| 2 | 8.934 | 808 | 797 | 2,4-Dimethylheptane | C9H20 | HC | - | - | - | - | - | 0.14 (6) |
| 3 | 9.273 | 823 | 821 | Acetone | C3H6O | CC | - | - | - | - | - | 0.26 (4) |
| 4 | 10.122 | 857 | 852 | 4-Methyloctane | C9H20 | HC | - | - | - | - | - | 0.19 (12) |
| 5 | 10.696 | 878 | 877 | 2-Methylfuran | C5H6O | FC | - | - | - | - | - | 0.08 (16) |
| 6 | 10.879 | 885 | 885 | 2,4-Dimethyl-1-heptene | C9H18 | HC | - | - | - | - | - | 0.62 (18) |
| 7 | 11.083 | 892 | 892 | Ethyl acetate | C4H8O2 | E | 29.10 (8) | - | - | - | - | - |
| 8 | 12.119 | 923 | 931 | 2-Methyl-butanal | C5H10O | CC | - | - | - | - | - | 0.27 (8) |
| 9 | 12.267 | 927 | 936 | 3-Methyl-butanal | C5H10O | CC | - | - | - | - | - | 0.34 (3) |
| 10 | 12.731 | 939 | 941 | Ethyl alcohol | C2H6O | A | 2.94 (24) | 5.81 (23) | 11.64 (7) | - | 0.77 (10) | 0.38 (47) |
| 11 | 13.450 | 957 | 957 | 2,2,4,6,6-Pentamethyl-heptane | C12H26 | HC | - | 0.90 (23) | - | - | - | 0.63 (5) |
| 12 | 13.650 | 962 | 962 | 2-Ethylfuran | C6H8O | FC | - | - | - | - | - | 0.07 (23) |
| 13 | 15.035 | 994 | 982 | Methyl butyrate | C5H10O2 | E | - | - | - | - | - | - |
| 14 | 15.269 | 999 | 1005 | 4-Methyl decane | C11H24 | HC | - | - | - | - | - | 0.39 (9) |
| 15 | 16.679 | 1028 | 1028 | α-Pinene | C10H16 | T | 6.19 (9) | 0.55 (18) | 58.06 (14) | 5.36 (17) | 167.48 (18) | 0.35 (8) |
| 16 | 16.868 | 1032 | 1032 | α-Thujene | C10H16 | T | 0.74 (6) | - | 20.84 (12) | 1.06 (15) | 4.95 (17) | 0.21 (25) |
| 17 | 17.826 | 1050 | 1050 | Toluene | C7H8 | HC | 12.05 (13) | - | - | 2.24 (9) | 1.29 (13) | - |
| 18 | 18.513 | 1063 | 1064 | α-Fenchene | C10H16 | T | - | - | - | - | 1.26 (8) | - |
| 19 | 18.906 | 1070 | 1070 | Camphene | C10H16 | T | - | - | 4.04 (14) | 0.75 (16) | 6.11 (10) | - |
| 20 | 20.042 | 1089 | 1088 | Hexanal | C6H12O | CC | - | - | - | 0.38 (3) | - | 0.78 (8) |
| 21 | 20.840 | 1108 | 1109 | 2-Methyl-2-butenal | C5H8O | CC | - | - | - | 1.92 (5) | - | - |
| 22 | 20.794 | 1107 | 1105 | β-Pinene | C10H16 | T | 3.11 (11) | 0.92 (23) | 1432.11 (10) | 40.10 (14) | 481.48 (7) | 0.80 (19) |
| 23 | 21.546 | 1121 | 1121 | Sabinene | C10H16 | T | 0.69 (9) | - | 94.76 (10) | 2.02 (15) | 20.54 (5) | 0.60 (6) |
| 24 | 22.069 | 1130 | 1128 | 4-Carene | C10H16 | T | 1.11 (15) | - | - | - | 1.48 (17) | - |
| 25 | 23.197 | 1150 | 1140 | (+)-3-Carene | C10H16 | T | 10.50 (6) | - | 5.71 (11) | 0.59 (19) | 1292.60 (1) | 0.54 (32) |
| 26 | 23.424 | 1154 | 1154 | 1-Methyl pyrrole | C5H7N | NC | - | - | - | - | - | 0.76 (25) |
| 27 | 24.008 | 1164 | 1160 | β-Myrcene | C10H16 | T | 5.28 (6) | - | 113.03 (10) | 11.39 (8) | 193.81 (8) | 0.42 (9) |
| 28 | 24.297 | 1168 | 1168 | α-Phellandrene | C10H16 | T | 121.53 (10) | - | 9.26 (10) | 10.73 (14) | 270.18 (1) | 0.27 (5) |
| 29 | 25.161 | 1182 | 1182 | Cumene | C9H12 | T | - | - | 1.23 (12) | 0.25 (20) | - | - |
| 30 | 25.199 | 1183 | 1183 | α-Terpinene | C10H16 | T | 4.26 (8) | - | 4.77 (9) | 1.47 (4) | 10.14 (11) | 0.50 (14) |
| 31 | 26.397 | 1201 | 1202 | Limonene | C10H16 | T | 17.77 (6) | 0.69 (18) | 37.73 (10) | 9.19 (11) | 1217.49 (2) | 1.76 (14) |
| 32 | 27.087 | 1214 | 1212 | β-Phellandrene | C10H16 | T | 6.77 (22) | - | 38.71 (10) | 2.75 (5) | 45.95 (2) | 0.49 (10) |
| 33 | 27.229 | 1217 | 1217 | Eucalyptol | C10H18O | T | 49.35 (17) | 1.12 (10) | 28.08 (23) | 2.65 (17) | - | - |
| 34 | 28.224 | 1234 | 1234 | 2-Hexenal | C6H10O | CC | - | - | - | 0.40 (1) | - | - |
| 35 | 28.528 | 1240 | 1241 | 2-Pentylfuran | C9H14O | FC | - | - | - | - | - | 0.45 (12) |
| 36 | 29.355 | 1254 | 1254 | γ-Terpinene | C10H16 | T | 4.79 (6) | 1.01 (22) | 2824.09 (7) | - | - | 2.19 (5) |
| 37 | 29.564 | 1257 | 1257 | β-Ocimene isomer | C10H16 | T | 0.54 (23) | - | - | 0.63 (6) | 1.92 (5) | 0.20 (18) |
| 38 | 29.140 | 1250 | 1250 | β-Phellandrene | C10H16 | T | - | - | - | - | 8.87 (3) | - |
| 39 | 29.713 | 1260 | 1261 | γ-Terpinene | C10H16 | T | - | - | - | 115.03 (12) | 20.46 (5) | - |
| 40 | 29.613 | 1258 | 1259 | α-Ocimene | C10H16 | T | - | - | - | - | 6.03 (6) | - |
| 41 | 30.476 | 1272 | 1275 | Styrene | C8H8 | HC | 0.26 (22) | 13.38 (13) | - | - | 0.75 (9) | 0.08 (23) |
| 42 | 31.088 | 1282 | 1284 | p-cymene | C10H14 | T | 78.13 (12) | 1.72 (21) | 1136.25 (6) | 109.60 (9) | 234.45 (3) | 2.65 (16) |
| 43 | 31.738 | 1293 | 1293 | Terpinolene | C10H16 | T | 37.49 (12) | - | 4.19 (7) | 4.54 (23) | 117.72 (17) | 0.25 (20) |
| 44 | 32.062 | 1298 | 1298 | Octanal | C8H16O | CC | 1.96 (14) | - | - | - | - | - |
| 45 | 34.297 | 1337 | 1336 | 2,2,6-Trimethylcyclohexanone | C9H16O | T | - | - | - | - | - | 0.23 (23) |
| 46 | 34.571 | 1342 | 1340 | 2-Heptenal isomer | C7H12O | CC | - | - | 2.72 (10) | - | - | - |
| 47 | 35.088 | 1351 | 1342 | 6-Methyl-5-hepten-2-one | C8H14O | CC | 1.06 (10) | 3.95 (5) | - | 0.73 (7) | - | 0.50 (11) |
| 48 | 36.638 | 1377 | 1385 | 3-Methylstyrene | C9H10 | HC | 0.50 (5) | - | - | - | - | - |
| 49 | 36.718 | 1378 | 1372 | Allyl isothiocyanate | C4H5NS | SC | - | - | - | 17.63 (8) | - | - |
| 50 | 37.959 | 1398 | 1400 | Tetradecane | C14H30 | HC | - | 0.58 (11) | - | 0.90 (1) | - | - |
| 51 | 39.109 | 1419 | 1414 | Fenchone | C10H16O | T | - | - | - | - | 0.62 (12) | - |
| 52 | 40.632 | 1446 | 1431 | 3-Ethyl-o-xylene | C10H14 | HC | - | 0.91 (6) | - | - | - | - |
| 53 | 41.116 | 1454 | 1463 | β-Ionone | C13H20O | T | - | 7.51 (9) | - | - | - | - |
| 54 | 41.138 | 1455 | 1456 | Dehydro-p-cymene | C10H14 | T | 13.29 (11) | - | 4.66 (8) | 3.54 (8) | 19.98 (16) | - |
| 55 | 41.246 | 1457 | 1456 | 1,2,3,4-Tetramethyl-benzene | C10H14 | HC | - | 1.04 (7) | - | - | - | - |
| 56 | 42.107 | 1471 | 1467 | α-Cubebene | C15H24 | ST | - | - | - | - | 31.46 (2) | - |
| 57 | 42.793 | 1483 | 1482 | δ-Elemene | C15H24 | ST | 3.14 (13) | 4.11 (16) | - | - | 598.43 (16) | - |
| 58 | 43.025 | 1487 | 1467 | Tetramethyl-pyrazine | C8H12N2 | NC | 3.96 (16) | - | - | 1.17 (3) | - | 0.19 (10) |
| 59 | 43.496 | 1495 | 1488 | a-Patchoulene | C15H24 | ST | - | - | - | - | 3.51 (4) | - |
| 60 | 43.815 | 1500 | 1491 | Ylangene | C15H24 | ST | - | 9.28 (18) | - | - | 1.22 (9) | - |
| 61 | 43.901 | 1502 | 1492 | Cyclosativene | C15H24 | ST | - | 51.44 (15) | - | - | 5.74 (13) | - |
| 62 | 44.240 | 1508 | 1509 | Copaene | C15H24 | ST | 3.72 (14) | 1064.64 (13) | 60.09 (12) | 5.63 (10) | 386.46 (12) | 0.35 (3) |
| 63 | 45.528 | 1534 | 1531 | β-Bourbonene | C15H24 | ST | 0.84 (24) | - | - | - | - | - |
| 64 | 45.807 | 1539 | 1544 | Calarene isomer | C15H24 | ST | 1.72 (22) | - | - | - | - | - |
| 65 | 46.168 | 1546 | 1540 | Camphor | C10H16O | T | - | - | 3.88 (8) | 23.57 (9) | 5.76 (8) | 0.14 (16) |
| 66 | 46.308 | 1549 | 1562 | α-Cedrene | C15H24 | ST | 1.03 (23) | - | - | - | - | - |
| 67 | 46.424 | 1551 | 1558 | Benzaldehyde | C7H6O | CC | 3.76 (21) | 85.40 (5) | - | - | - | 0.88 (2) |
| 68 | 46.491 | 1552 | 1552 | Linalool | C10H18O | T | - | - | - | 445.08 (10) | 91.33 (9) | - |
| 69 | 46.776 | 1557 | 1558 | β cubebene | C15H24 | ST | - | - | - | - | 46.72 (19) | - |
| 70 | 46.820 | 1558 | 1554 | Calarene isomer | C15H24 | ST | - | 32.50 (14) | - | - | - | - |
| 71 | 46.930 | 1560 | 1563 | β-Terpineol | C10H18O | T | - | - | 36.07 (9) | 2.22 (22) | - | 0.44 (25) |
| 72 | 47.032 | 1562 | 1559 | Isoledene | C15H24 | ST | - | 5.18 (13) | - | - | - | - |
| 73 | 47.215 | 1566 | 1568 | Linalyl acetate | C12H20O2 | E | - | - | - | - | - | 0.54 (21) |
| 74 | 47.353 | 1568 | 1552 | Aristolene | C15H24 | ST | 11.37 (7) | - | - | - | - | - |
| 75 | 48.242 | 1585 | 1585 | α-Bergamotene | C15H24 | ST | - | 9.54 (24) | - | - | - | - |
| 76 | 48.561 | 1590 | 1597 | α-Santalene | C15H24 | ST | - | 8.49 (16) | - | - | - | - |
| 77 | 48.577 | 1591 | 1578 | p-Menth-2-en-1-ol isomer | C10H18O | T | 3.09 (13) | - | - | - | - | - |
| 78 | 48.736 | 1593 | 1582 | Pinocamphone | C10H16O | T | - | - | 516.77 (3) | - | - | - |
| 79 | 48.813 | 1595 | 1601 | (-)-Clovene | C15H24 | ST | 2.26 (11) | 17.26 (12) | - | - | - | - |
| 80 | 48.972 | 1598 | 1602 | a-Elemene | C15H24 | ST | - | 11.23 (21) | - | - | 17.39 (13) | - |
| 81 | 49.107 | 1600 | 1590 | Bergamotene isomer | C15H24 | ST | 5.11 (3) | 39.59 (17) | - | 2.46 (6) | 1.89 (16) | - |
| 82 | 49.120 | 1600 | 1584 | Isobornyl acetate | C12H20O2 | E | - | - | 47.68 (5) | - | - | - |
| 83 | 49.554 | 1605 | 1605 | β-Elmene | C15H24 | ST | - | 45.70 (14) | - | - | 127.48 (16) | - |
| 84 | 49.624 | 1606 | 1606 | 2-Undecanone | C11H22O | CC | 2.72 (19) | - | - | - | - | - |
| 85 | 50.032 | 1610 | 1619 | (+)-Longifolene | C15H24 | ST | - | 6.43 (17) | - | - | - | - |
| 86 | 50.060 | 1610 | 1606 | (-)-Terpinen-4-ol | C10H18O | T | - | - | 13.46 (3) | 2.66 (9) | 10.13 (14) | 0.61 (4) |
| 87 | 50.303 | 1613 | 1612 | Caryophyllene isomer | C15H24 | ST | 123.13 (7) | 37.67 (17) | 59.95 (3) | 9.71 (9) | 3157.13 (13) | 0.64 (15) |
| 88 | 50.794 | 1618 | 1625 | (+)-Aromadendrene | C15H24 | ST | - | 4.98 (18) | - | - | - | - |
| 89 | 50.953 | 1620 | 1626 | α-Cedrene | C15H24 | ST | - | 3.35 (19) | - | - | - | - |
| 90 | 51.173 | 1622 | 1621 | cis-p-2-menthen-1-ol | C10H18O | T | - | - | 5.82 (8) | - | - | - |
| 91 | 51.274 | 1623 | 1635 | γ-Gurjunene | C15H24 | ST | - | 3.24 (23) | - | - | - | - |
| 92 | 51.540 | 1626 | 1624 | β-Cyclocitral | C10H16O | T | - | - | - | - | - | 0.31 (6) |
| 93 | 52.138 | 1632 | 1634 | Myrtenal | C10H14O | T | - | - | 6.09 (1) | - | - | - |
| 94 | 52.610 | 1637 | 1653 | (-)-β-Santalene | C15H24 | ST | - | 11.83 (22) | - | - | 18.37 (4) | - |
| 95 | 52.683 | 1637 | 1640 | Pinocarveol | C10H16O | T | - | - | 7.45 (6) | - | - | - |
| 96 | 52.870 | 1639 | 1633 | Farnesene isomer | C15H24 | ST | 34.21 (9) | - | 78.79 (5) | 4.43 (14) | 17.53 (7) | - |
| 97 | 53.172 | 1642 | 1645 | Acetophenone | C8H8O | CC | - | 73.03 (7) | - | - | - | - |
| 98 | 53.189 | 1643 | 1640 | β-Patchoulene | C15H24 | ST | - | - | 8.13 (9) | - | - | - |
| 99 | 53.320 | 1644 | 1652 | α-Humulene | C15H24 | ST | - | - | - | 0.98 (6) | 6.74 (13) | - |
| 100 | 53.723 | 1648 | 1652 | α-Guaiene | C15H24 | ST | - | - | 12.04 (5) | - | - | - |
| 101 | 53.774 | 1648 | 1652 | Estragole | C10H12O | T | - | - | - | - | 17.26 (7) | - |
| 102 | 53.857 | 1649 | 1662 | Pulegone | C10H16O | T | - | - | - | 1.02 (1) | - | - |
| 103 | 53.920 | 1650 | 1654 | β-Humulene | C15H24 | ST | - | 7.15 (14) | - | - | - | - |
| 104 | 54.063 | 1651 | 1672 | Epizonarene | C15H24 | ST | 74.63 (5) | 55.35 (18) | 6.78 (3) | 3.21 (2) | 264.01 (15) | - |
| 105 | 54.455 | 1704 | 1697 | Farnesene isomer | C15H24 | ST | 7.06 (13) | - | - | - | - | - |
| 106 | 54.586 | 1707 | 1689 | β-Panasinsene | C15H24 | ST | 24.62 (1) | - | - | - | - | - |
| 107 | 54.755 | 1710 | 1710 | α-Terpineol | C10H18O | T | 6.48 (18) | - | 21.55 (7) | 5.15 (5) | 49.27 (14) | 0.26 (15) |
| 108 | 54.812 | 1712 | 1725 | γ-Muurolene | C15H24 | ST | - | 227.05 (20) | - | - | - | - |
| 109 | 55.011 | 1716 | 1720 | β-Cadinene | C15H24 | ST | - | - | - | - | 11.22 (9) | - |
| 110 | 55.120 | 1718 | 1707 | Zonarene | C15H24 | ST | - | - | 78.06 (5) | 5.23 (10) | - | - |
| 111 | 55.256 | 1721 | 1721 | γ-Himachalene | C15H24 | ST | - | 29.13 (23) | - | - | - | - |
| 112 | 55.287 | 1721 | 1726 | Valencene | C15H24 | ST | - | - | - | - | 15.33 (14) | - |
| 113 | 55.336 | 1722 | 1731 | (-)-Zingiberene | C15H24 | ST | - | - | 37.51 (6) | 2.00 (13) | - | - |
| 114 | 55.905 | 1734 | 1736 | Bicyclogermacrene | C15H24 | ST | - | 81.26 (23) | - | - | - | - |
| 115 | 56.093 | 1738 | 1749 | (-)-δ-Cadinene | C15H24 | ST | 450.35 (7) | - | - | 16.82 (10) | - | - |
| 116 | 56.109 | 1738 | 1731 | α-Selinene | C15H24 | ST | - | - | - | - | 70.59 (4) | - |
| 117 | 56.386 | 1744 | 1741 | β-Bisabolene | C15H24 | ST | 134.58 (6) | 109.30 (24) | 14.80 (12) | 5.11 (11) | 26.80 (15) | 0.28 (1) |
| 118 | 56.543 | 1747 | 1750 | α-Muurolene | C15H24 | ST | - | 473.10 (23) | - | - | 8.15 (5) | - |
| 119 | 56.737 | 1751 | 1742 | β-Selinene | C15H24 | ST | - | - | - | - | 102.21 (16) | - |
| 120 | 56.828 | 1753 | 1733 | Citral isomer | C10H16O | T | - | - | - | - | - | 0.40 (17) |
| 121 | 56.858 | 1753 | 1779 | γ-Selinene | C15H24 | ST | - | 37.63 (21) | - | - | 69.06 (17) | - |
| 122 | 56.922 | 1754 | 1759 | Phellandral | C10H16O | T | - | - | 97.64 (4) | 3.14 (14) | - | - |
| 123 | 57.037 | 1757 | 1760 | (+)-Epi-bicyclosesquiphellandrene | C15H24 | ST | 25.14 (15) | - | - | - | - | - |
| 124 | 57.317 | 1762 | 1778 | Germacrene B | C15H24 | ST | - | - | - | - | 18.87 (13) | - |
| 125 | 57.45 | 1765 | 1765 | Citronellol | C10H20O | T | - | - | - | 26.91 (10) | - | - |
| 126 | 58.177 | 1779 | 1749 | δ-Cadinene | C15H24 | ST | - | 717.65 (24) | - | - | 197.13 (12) | - |
| 127 | 58.551 | 1787 | 1786 | Bisabolene isomer | C15H24 | ST | - | 68.37 (24) | - | - | 12.53 (15) | - |
| 128 | 58.662 | 1789 | 1782 | β-Sesquiphellandrene | C15H24 | ST | 511.76 (10) | - | 3.91 (9) | 18.70 (9) | - | - |
| 129 | 58.812 | 1792 | 1777 | α-Curcumene | C15H22 | ST | 817.86 (8) | 80.41 (22) | 4.48 (18) | 27.17 (8) | 7.76 (8) | - |
| 130 | 59.432 | 1804 | 1802 | Cuminaldehyde | C10H12O | T | 27.33 (14) | 5.69 (24) | 9694.84 (3) | 777.58 (10) | 24.54 (15) | 1.40 (20) |
| 131 | 59.548 | 1807 | 1813 | Methyl salicylate | C8H8O3 | E | - | 21.04 (23) | - | - | 12.70 (13) | - |
| 132 | 59.972 | 1817 | 1840 | α-Panasinsene | C15H24 | ST | - | 19.58 (20) | - | - | - | - |
| 133 | 60.283 | 1824 | 1821 | Neryl isobutyrate | C14H20O2 | E | - | - | - | - | 22.10 (14) | - |
| 134 | 60.287 | 1824 | 1847 | Cis-carveol | C10H16O | T | 8.86 (15) | - | - | - | - | - |
| 135 | 61.508 | 1851 | 1851 | Geraniol | C10H18O | T | 9.87 (19) | - | - | - | - | 1.06 (21) |
| 136 | 61.561 | 1853 | 1845 | Anethole | C10H12O | T | - | 5.08 (25) | 98.43 (9) | 10.09 (3) | 5.58 (15) | - |
| 137 | 61.705 | 1856 | 1868 | Thymol acetate | C12H16O2 | E | - | - | - | - | 7.23 (15) | - |
| 138 | 61.978 | 1862 | 1859 | Calamenene | C15H22 | ST | - | 420.90 (24) | - | - | 26.87 (13) | - |
| 139 | 62.036 | 1863 | 1861 | 2,4-Dimethylacetophenone | C10H12O | CC | 27.51 (15) | - | - | 2.84 (21) | - | - |
| 140 | 63.653 | 1898 | 1902 | Geranyl butyrate | C14H24O2 | E | 25.14 (9) | - | - | 2.55 (16) | - | - |
| 141 | 63.944 | 1905 | 1903 | Safrole | C10H10O2 | T | - | - | - | - | 2.31 (8) | - |
| 142 | 65.943 | 1951 | 1926 | α-Calacorene | C15H20 | ST | - | 94.52 (24) | - | - | 3.87 (3) | - |
| 143 | 66.413 | 1962 | 1962 | 2-(4-Methylphenyl)propan-1-ol | C10H14O | A | - | - | - | - | 10.56 (12) | - |
| 144 | 66.688 | 1968 | 1975 | Jasmone | C11H16O | T | - | - | - | - | - | 0.52 (15) |
| 145 | 67.640 | 1990 | 1996 | 4-Methylguaiacol | C8H10O2 | VP | - | - | - | 2.28 (17) | - | - |
| 146 | 68.058 | 1999 | 2009 | Nerolidol | C15H26O | ST | - | - | 12.66 (3) | - | - | - |
| 147 | 69.278 | 2009 | 2008 | Caryophyllene oxide | C15H24O | ST | 29.20 (11) | - | - | - | 84.25 (12) | - |
| 148 | 69.449 | 2010 | 2012 | Methyl eugenol | C11H14O2 | VP | - | 146.68 (23) | 121.21 (46) | - | - | - |
| 149 | 71.220 | 2023 | 2018 | Germacrene D-4-ol | C15H26O | ST | - | - | 58.67 (4) | - | - | - |
| 150 | 71.667 | 2026 | 2017 | Cinnamaldehyde | C9H8O | CC | - | 6996.90 (24) | 84.27 (41) | - | 7.77 (9) | 1.09 (11) |
| 151 | 72.319 | 2030 | 2036 | Cadinol | C15H26O | ST | 12.26 (16) | - | - | - | - | - |
| 152 | 72.429 | 2031 | 2033 | o-Cresol | C7H8O | VP | - | - | - | - | 6.39 (11) | - |
| 153 | 73.253 | 2137 | 2143 | γ-Eudesmol | C15H26O | ST | - | - | 389.01 (4) | 26.61 (8) | - | - |
| 154 | 74.492 | 2145 | 2166 | Spathulenol | C15H24O | ST | - | - | - | - | 4.71 (10) | - |
| 155 | 75.544 | 2152 | 2150 | Eugenol | C10H12O2 | VP | - | - | 23.05 (13) | - | - | 1.93 (20) |
| 156 | 77.911 | 2168 | 2157 | Thymol | C10H14O | VP | - | - | 26.30 (18) | - | 7.52 (13) | 1.64 (6) |
| 157 | 77.914 | 2168 | 2173 | Carvacrol | C10H14O | VP | 16.23 (16) | - | - | - | - | - |
| 158 | 78.182 | 2170 | 2175 | α-Bisabolol | C15H26O | ST | - | 14.02 (22) | - | - | - | - |
| 159 | 79.296 | 2177 | 2177 | τ-Muurolol | C15H26O | ST | - | 8.19 (21) | - | - | - | - |
| 160 | 79.587 | 2179 | 2188 | Cadalene | C15H18 | ST | - | 31.97 (24) | - | - | - | - |
| 161 | 80.607 | 2185 | 2187 | Dihydrojuneol | C15H28O | ST | 1447.25 (8) | - | - | 92.27 (15) | - | - |
| 162 | 81.898 | 2193 | 2209 | Cadinol | C15H26O | ST | - | 19.41 (21) | - | - | - | - |
| Peak n° | Volatile Organic Metabolites | Potential Bioactive Effects 1 | Spices | References | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Antibacterial | Antidiabetic | Anti-inflammatory | Antifungal | Antioxidant | Antiproliferative | Antitumor | Cytotoxic | ||||
| 22 | β-Pinene | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | [4,11,33,39,40] | ||||
| 25 | (+)-3-Carene | x | x | x | Saffron, cumin, curry, black pepper and sweet paprika | ||||||
| 27 | β-Myrcene | x | x | x | x | x | x | x | Saffron, cumin, curry, black pepper and sweet paprika | ||
| 31 | Limonene | x | x | x | x | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | |
| 36 | γ-Terpinene | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | |||||
| 42 | p-Cymene | x | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | ||||
| 68 | Linalool | x | x | x | x | x | Curry and black pepper | ||||
| 78 | Pinocamphone | x | x | x | x | x | Cumin | ||||
| 87 | Caryophyllene isomer | x | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | ||||
| 118 | α-Muurolene | x | x | Cinnamon and black pepper | |||||||
| 128 | β-Sesquiphellandrene | x | x | x | x | x | Saffron, cumin and curry | ||||
| 129 | α-Curcumene | x | x | Saffron, cinnamon, cumin, curry and black pepper | |||||||
| 130 | Cuminaldehyde | x | x | x | x | x | x | Saffron, cinnamon, cumin, curry, black pepper and sweet paprika | |||
| 150 | Cynamaldehyde | x | x | x | x | x | x | Cinnamon, cumin, black pepper and sweet paprika | |||
| 155 | Eugenol | x | x | x | Cumin and sweet paprika | ||||||
| 156 | Thymol | x | x | x | x | Cumin, black pepper and sweet paprika | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Izcara, S.; Perestrelo, R.; Morante-Zarcero, S.; Sierra, I.; Câmara, J.S. Spices Volatilomic Fingerprinting—A Comprehensive Approach to Explore Its Authentication and Bioactive Properties. Molecules 2022, 27, 6403. https://doi.org/10.3390/molecules27196403
Izcara S, Perestrelo R, Morante-Zarcero S, Sierra I, Câmara JS. Spices Volatilomic Fingerprinting—A Comprehensive Approach to Explore Its Authentication and Bioactive Properties. Molecules. 2022; 27(19):6403. https://doi.org/10.3390/molecules27196403
Chicago/Turabian StyleIzcara, Sergio, Rosa Perestrelo, Sonia Morante-Zarcero, Isabel Sierra, and José S. Câmara. 2022. "Spices Volatilomic Fingerprinting—A Comprehensive Approach to Explore Its Authentication and Bioactive Properties" Molecules 27, no. 19: 6403. https://doi.org/10.3390/molecules27196403
APA StyleIzcara, S., Perestrelo, R., Morante-Zarcero, S., Sierra, I., & Câmara, J. S. (2022). Spices Volatilomic Fingerprinting—A Comprehensive Approach to Explore Its Authentication and Bioactive Properties. Molecules, 27(19), 6403. https://doi.org/10.3390/molecules27196403