Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism
Abstract
:1. Introduction
2. Ginger as a Functional Food
2.1. Nutritional Composition and Traditional Use
2.2. Phytochemistry and Health Benefits
2.3. The Growing Popularity of Ginger
2.4. Safety
2.5. Adverse Events
2.6. Drug Interactions
3. Pathophysiology of IDA and Its Treatment
3.1. Iron Homeostasis
3.2. IDA and Its Aetiology
3.3. Treatment of IDA
3.4. Adverse Effects of Oral Iron Therapy
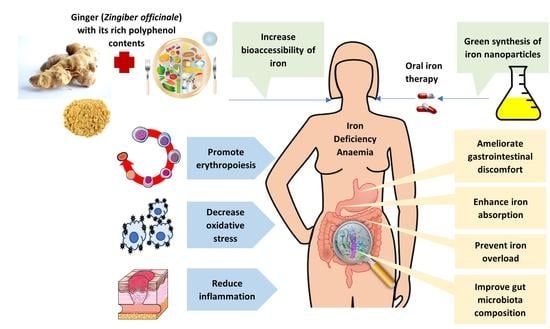
4. Ginger and IDA
4.1. Iron Absorption Enhancement
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Action
4.4. Gut Microbiota Modulation
4.5. Erythropoiesis Stimulation
4.6. Iron Overload Prevention
4.7. Ginger-Synthesised Iron Nanoparticles
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AI | Anaemia of inflammation |
| CAT | Catalase |
| CI | Confidence interval |
| CRP | C-reactive protein |
| DcytB | Duodenal cytochrome B |
| DMT-1 | Divalent metal transporter 1 |
| FeNP | Iron nanoparticle |
| FPN | Ferroportin |
| Gata1 | GATA-binding factor 1 |
| GR | Glutathione reductase |
| GST | Glutathione-S-transferase |
| HAMP | Hepcidin antimicrobial peptide |
| Hb | Haemoglobin |
| HSPC | Haematopoietic stem/progenitor cells |
| IDA | Iron deficiency anaemia |
| MDA | Malondialdehyde |
| NF | Nuclear factor kappa B |
| NO | Nitric oxide |
| NOAEL | No observed adverse effect level |
| RBC | Red blood cell |
| RCT | Randomised controlled trial |
| SF | Serum ferritin |
| siRNA | Short interference RNA |
| SOD | Superoxide dismutase |
| TNF | Tumour necrotic factor |
| WHO | World Health Organization |
Appendix A. International Patents
| Publication No | Date | Classification Code | Title | Country |
|---|---|---|---|---|
| 101243891 | 20 August 2008 | A23L 1/337 | Sea tangle vegetarian stuffing boiled dumplings and its processing method | China |
| 103947928 | 11 March 2014 | A23L 1/10 | Fleece-flower root nutrition eight-treasure porridge and its preparation method | China |
| 104026495 | 10 September 2014 | A23L 1/212 | Haw flake containing pig blood and coarse cereals, and preparation method thereof | China |
| 105495158 | 25 September 2014 | A23L 1/315 | Black-bone chicken sausage and preparation method thereof | China |
| 104095016 | 15 October 2014 | A61K 36/9068 | Infantile iron-deficiency anemia treating cookie and preparing method thereof | China |
| 104323303 | 4 November 2015 | A23L 1/314 | Method for making tomato beef stewed product | China |
| 104643216 | 27 May 2015 | A23L 2/02 | Blood-replenishing and beautifying calcium blended lotus root juice and preparation method thereof | China |
| 105664116 | 15 June 2016 | A61K 36/9068 | Traditional Chinese medicine for treating infant iron deficiency anemia as well as preparation method and application thereof | China |
| 106362108 | 1 February 2017 | A61K 36/9068 | Traditional Chinese medicinal pill used for hematogenesis | China |
| 106616937 | 10 May 2017 | A23L 31/00 | Stropharia rugosoannulata and black chicken can and preparation method thereof | China |
| 106889422 | 27 June 2017 | A61K 36/9068 | Edible flour for tonifying blood and warming the uterus and production method thereof | China |
| 107772293 | 9 March 2018 | A23L 13/50 | Body-nourishing black bone chicken | China |
| 107772373 | 9 March 2018 | A61K 36/9068 | Decoction preventing and curing osteoporosis | China |
| 108887607 | 27 November 2018 | A23L 13/70 | Spicy shredded pork with garlic sauce and making method thereof | China |
| 108433084 | 24 August 2018 | A23L 27/50 | Soy sauce | China |
| 113278488 | 20 August 2021 | A61K 36/9068 | Spartina alterniflora spleen-tonifying stomach-nourishing pericarpium citri reticulatae wine decocting pot and preparation process thereof | China |
References
- Chaparro, C.M.; Suchdev, P.S. Anemia epidemiology, pathophysiology, and etiology in low- and middle-income countries. Ann. N. Y. Acad. Sci. 2019, 1450, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.; Parsi, M.; Badireddy, M. Anemia. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK499994/ (accessed on 13 March 2022).
- Gardner, W.; Kassebaum, N. Global, regional, and national prevalence of anemia and its causes in 204 countries and territories, 1990–2019. Curr. Dev. Nutr. 2020, 4, 830. [Google Scholar] [CrossRef]
- Safiri, S.; Kolahi, A.A.; Noori, M.; Nejadghaderi, S.A.; Karamzad, N.; Bragazzi, N.L.; Sullman, M.J.M.; Abdollahi, M.; Collins, G.S.; Kaufman, J.S.; et al. Burden of anemia and its underlying causes in 204 countries and territories, 1990–2019: Results from the Global Burden of Disease Study 2019. J. Hematol. Oncol. 2021, 14, 185. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. World Health Statistics 2021: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Australian Bureau of Statistics. Australian Health Survey 2011–2012: Biomedical Results for Chronic Diseases. 2013. Available online: https://www.abs.gov.au/statistics/health/health-conditions-and-risks/australian-health-survey-biomedical-results-chronic-diseases/latest-release (accessed on 15 March 2022).
- Azzopardi, P.S.; Sawyer, S.M.; Carlin, J.B.; Degenhardt, L.; Brown, N.; Brown, A.D.; Patton, G.C. Health and wellbeing of indigenous adolescents in Australia: A systematic synthesis of population data. Lancet 2018, 391, 766–782. [Google Scholar] [CrossRef]
- Mantadakis, E.; Chatzimichael, E.; Zikidou, P. Iron deficiency anemia in children residing in high and low-income countries: Risk factors, prevention, diagnosis and therapy. Mediterr. J. Hematol. Infect. Dis. 2020, 12, e2020041. [Google Scholar] [CrossRef]
- Tolkien, Z.; Stecher, L.; Mander, A.P.; Pereira, D.I.; Powell, J.J. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: A systematic review and meta-analysis. PLoS ONE 2015, 10, 1–20. [Google Scholar] [CrossRef]
- Gereklioglu, C.; Asma, S.; Korur, A.; Erdogan, F.; Kut, A. Medication adherence to oral iron therapy in patients with iron deficiency anemia. Pak. J. Med. Sci. 2016, 32, 604–607. [Google Scholar] [CrossRef]
- World Flora Online (WFO). World Flora Online Taxonomic Backbone v.2022.04. 2022. Available online: http://www.worldfloraonline.org/downloadData (accessed on 2 September 2022).
- Wu, D. A preliminary study on the origin of ginger. Agric. Archaeol. 1985, 5, 247–250. [Google Scholar]
- The Observatory of Economic Complexity. Ginger (HS: 091010) Product Trade, Exporters and Importers. Available online: https://oec.world/en/profile/hs92/ginger (accessed on 10 March 2022).
- Syafitri, D.M.; Levita, J.; Mutakin, M.; Diantini, A. A review: Is ginger (Zingiber officinale var. Roscoe) potential for future phytomedicine? Indones. J. Appl. Sci. 2018, 8, 8–13. [Google Scholar] [CrossRef]
- Martirosyan, D.; Singharaj, B. Health claims and functional food: The future of functional foods under FDA and EFSA regulation. In Functional Foods for Chronic Diseases, 1st ed.; Martirosyan, D.M., Ed.; Food Science Publisher: Chicago, IL, USA, 2016; pp. 410–424. [Google Scholar]
- He, L.; Qin, Z.; Li, M.; Chen, Z.; Zeng, C.; Yao, Z.; Yu, Y.; Dai, Y.; Yao, X. Metabolic profiles of ginger, a functional food, and its representative pungent compounds in rats by ultraperformance liquid chromatography coupled with quadrupole time-of-flight tandem mass spectrometry. J. Agric. Food Chem. 2018, 66, 9010–9033. [Google Scholar] [CrossRef]
- Srivastava, N.; Singh, S.; Gupta, A.C.; Shanker, K.; Bawankule, D.U.; Luqman, S. Aromatic ginger (Kaempferia galanga L.) extracts with ameliorative and protective potential as a functional food, beyond its flavor and nutritional benefits. Toxicol. Rep. 2019, 6, 521–528. [Google Scholar] [CrossRef]
- Ozkur, M.; Benlier, N.; Takan, I.; Vasileiou, C.; Georgakilas, A.G.; Pavlopoulou, A.; Cetin, Z.; Saygili, E.I. Ginger for healthy ageing: A systematic review on current evidence of its antioxidant, anti-inflammatory, and anticancer properties. Oxidative Med. Cell. Longev. 2022, 2022, 1–16. [Google Scholar] [CrossRef]
- Ajayi, O.B.; Akomolafe, S.F.; Akinyemi, F.T. Food value of two varieties of ginger (Zingiber officinale) commonly consumed in Nigeria. ISRN Nutr. 2013, 2013, 359727. [Google Scholar] [CrossRef]
- Sangwan, A.; Kawatra, A.; Sehgal, S. Nutritional composition of ginger powder prepared using various drying methods. J. Food Sci. Technol. 2014, 51, 2260–2262. [Google Scholar] [CrossRef]
- Bode, A.M.; Dong, Z. The amazing and mighty ginger. In Herbal Medicine: Biomolecular and Clinical Aspects, 2nd ed.; Benzie, I.F.F., Wachtel-Galor, S., Eds.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2011. Available online: https://www.ncbi.nlm.nih.gov/books/NBK92775/ (accessed on 2 September 2022).
- Li, X.; Ao, M.; Zhang, C.; Fan, S.; Chen, Z.; Yu, L. Zingiberis rhizoma recens: A review of its traditional uses, phytochemistry, pharmacology, and toxicology. Evid.-Based Complement. Altern. Med. 2021, 2021, 6668990. [Google Scholar] [CrossRef]
- Unuofin, J.O.; Masuku, N.P.; Paimo, O.K.; Lebelo, S.L. Ginger from farmyard to town: Nutritional and pharmacological applications. Front. Pharmacol. 2021, 12, 779352. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.; Luo, D.; Ma, Y.; Zhang, J.; Li, M.; Yao, L.; Shi, X.; Liu, X.; Yang, K. Ginger for health care: An overview of systematic reviews. Complement. Ther. Med. 2019, 45, 114–123. [Google Scholar] [CrossRef]
- Anh, N.H.; Kim, S.J.; Long, N.P.; Min, J.E.; Yoon, Y.C.; Lee, E.G.; Kim, M.; Kim, T.J.; Yang, Y.Y.; Son, E.Y.; et al. Ginger on human health: A comprehensive systematic review of 109 randomized controlled trials. Nutrients 2020, 12, 157. [Google Scholar] [CrossRef]
- de Lima, R.M.T.; Reis, A.C.D.; de Menezes, A.A.P.M.; de Oliveira Santos, J.V.; de Oliveira Filho, J.W.G.; de Oliveira Ferreira, J.R.; de Alencar, M.V.O.B.; da Mata, A.M.O.F.; Khan, I.N.; Islam, A.; et al. Protective and therapeutic potential of ginger (Zingiber officinale) extract and [6]-gingerol in cancer: A comprehensive review. Phytother. Res. 2018, 32, 1885–1907. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, J.; Zhang, Y. Research progress on chemical constituents of Zingiber officinale Roscoe. BioMed Res. Int. 2019, 2019, 5370823. [Google Scholar] [CrossRef]
- Mao, Q.Q.; Xu, X.Y.; Cao, S.Y.; Gan, R.Y.; Corke, H.; Beta, T.; Li, H.B. Bioactive compounds and bioactivities of ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef] [Green Version]
- Rahmani, A.H.; Shabrmi, F.M.A.; Aly, S.M. Active ingredients of ginger as potential candidates in the prevention and treatment of diseases via modulation of biological activities. In Int. J. Physiol. Pathophysiol. Pharmacol.; 2014; 6, pp. 125–136. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4106649/ (accessed on 2 September 2022).
- Hasan, H.A.; Raauf, A.M.R.; Razik, B.M.A.; Hassan, B.A.R. Chemical composition and antimicrobial activity of the crude extracts isolated from Zingiber officinale by different solvents. Pharm. Anal. Acta 2012, 3, 2. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Identification and concentration of some flavonoid components in malaysian young ginger (Zingiber officinale Roscoe) varieties by a high performance liquid chromatography method. Molecules 2010, 15, 6231–6243. [Google Scholar] [CrossRef]
- Rahman, S.; Salehin, F.; Iqbal, A. In vitro antioxidant and anticancer activity of young Zingiber officinale against human breast carcinoma cell lines. BMC Complement. Altern. Med. 2011, 11, 76. [Google Scholar] [CrossRef]
- Fahmi, A.; Hassanen, N.; Abdur-Rahman, M.; Shams-Eldin, E. Phytochemicals, antioxidant activity and hepatoprotective effect of ginger (Zingiber officinale) on diethylnitrosamine toxicity in rats. Biomarkers 2019, 24, 436–447. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.; Rahmat, A. Synthesis of phenolics and flavonoids in ginger (Zingiber officinale Roscoe) and their effects on photosynthesis rate. Int. J. Mol. Sci. 2010, 11, 4539–4555. [Google Scholar] [CrossRef]
- Ghasemzadeh, A.; Jaafar, H.Z.E.; Rahmat, A. Variation of the Phytochemical Constituents and Antioxidant Activities of Zingiber officinale var. rubrum Theilade Associated with Different Drying Methods and Polyphenol Oxidase Activity. Molecules 2016, 21, 780. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Wang, X.; Ji, R.; Liu, L.; Qiao, Y.; Lou, Z.; Ma, C.; Li, S.; Wang, H.; Ho, C.T. Occurrence, biological activity and metabolism of 6-shogaol. Food Funct. 2018, 9, 1310–1327. [Google Scholar] [CrossRef] [PubMed]
- Sang, S.; Snook, H.D.; Tareq, F.S.; Fasina, Y. Precision research on ginger: The type of ginger matters. J. Agric. Food Chem. 2020, 68, 8517–8523. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.G.; Kim, S.Y.; Jeong, M.; Oh, M.S. Pharmacotherapeutic potential of ginger and its compounds in age-related neurological disorders. Pharmacol. Ther. 2018, 182, 56–69. [Google Scholar] [CrossRef]
- Sampath, C.; Sang, S.; Ahmedna, M. In vitro and in vivo inhibition of aldose reductase and advanced glycation end products by phloretin, epigallocatechin 3-gallate and [6]-gingerol. Biomed. Pharmacother. 2016, 84, 502–513. [Google Scholar] [CrossRef]
- Sampath, C.; Rashid, M.R.; Sang, S.; Ahmedna, M. Specific bioactive compounds in ginger and apple alleviate hyperglycemia in mice with high fat diet-induced obesity via Nrf2 mediated pathway. Food Chem. 2017, 226, 79–88. [Google Scholar] [CrossRef]
- Ahmad, B.; Rehman, M.U.; Amin, I.; ur Rahman Mir, M.; Ahmad, S.B.; Farooq, A.; Muzamil, S.; Hussain, I.; Masoodi, M.; Fatima, B. Zingerone (4-(4-hydroxy-3-methylphenyl) butan-2-one) protects against alloxan-induced diabetes via alleviation of oxidative stress and inflammation: Probable role of NF-kB activation. Saudi Pharm. J. 2018, 26, 1137–1145. [Google Scholar] [CrossRef]
- Cheng, Q.; Feng, X.; Meng, Q.; Li, Y.; Chen, S.; Wang, G.; Nie, K. [6]-gingerol ameliorates cisplatin-induced PICA by regulating the TPH/MAO-A/SERT/5-HT/5-HT3 receptor system in rats. Drug Des. Dev. Ther. 2020, 14, 4085–4099. [Google Scholar] [CrossRef]
- Qian, Q.H.; Yue, W.; Chen, W.H.; Yang, Z.H.; Liu, Z.T.; Wang, Y.X. Effect of gingerol on substance P and NK_1 receptor expression in a vomiting model of mink. Chin. Med. J. 2010, 123, 478–484. [Google Scholar]
- Qian, Q.; Yue, W.; Wang, Y.; Yang, Z.; Liu, Z.; Chen, W. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch. Pharmacal Res. 2009, 32, 565–573. [Google Scholar] [CrossRef]
- Qian, W.; Cai, X.; Wang, Y.; Zhang, X.; Zhao, H.; Qian, Q.; Yang, Z.; Liu, Z.; Hasegawa, J. Effect of gingerol on cisplatin-induced pica analogous to emesis via modulating expressions of dopamine 2 receptor, dopamine transporter and tyrosine hydroxylase in the vomiting model of rats. Yonago Acta Med. 2016, 59, 100–110. [Google Scholar]
- Tian, L.; Qian, W.; Qian, Q.; Zhang, W.; Cai, X. Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J. Nat. Med. 2020, 74, 353–370. [Google Scholar] [CrossRef]
- Tzeng, T.F.; Chang, C.J.; Liu, I.M. 6-gingerol inhibits rosiglitazone-induced adipogenesis in 3T3-L1 adipocytes. Phytother. Res. 2014, 28, 187–192. [Google Scholar] [CrossRef]
- Saravanan, G.; Ponmurugan, P.; Deepa, M.A.; Senthilkumar, B. Anti-obesity action of gingerol: Effect on lipid profile, insulin, leptin, amylase and lipase in male obese rats induced by a high-fat diet. J. Sci. Food Agric. 2014, 94, 2972–2977. [Google Scholar] [CrossRef]
- Suk, S.; Seo, S.G.; Yu, J.G.; Yang, H.; Jeong, E.; Jang, Y.J.; Yaghmoor, S.S.; Ahmed, Y.; Yousef, J.M.; Abualnaja, K.O.; et al. A bioactive constituent of ginger, 6-shogaol, prevents adipogenesis and stimulates lipolysis in 3T3-L1 adipocytes. J. Food Biochem. 2016, 40, 84–90. [Google Scholar] [CrossRef]
- Choi, J.; Kim, K.J.; Kim, B.H.; Koh, E.J.; Seo, M.J.; Lee, B.Y. 6-gingerol suppresses adipocyte-derived mediators of inflammation in vitro and in high-fat diet-induced obese zebra fish. Planta Med. 2017, 83, 245–253. [Google Scholar] [CrossRef]
- Sampath, S.J.P.; Birineni, S.; Perugu, S.; Kotikalapudi, N.; Venkatesan, V. Therapeutic efficacy of 6-gingerol and 6-shogaol in promoting browning of white adipocytes vis-à-vis enhanced thermogenesis portrayed in high fat milieu. Food Biosci. 2021, 42, 101211. [Google Scholar] [CrossRef]
- Zhang, F.L.; Zhou, B.W.; Yan, Z.Z.; Zhao, J.; Zhao, B.C.; Liu, W.F.; Li, C.; Liu, K.X. 6-gingerol attenuates macrophages pyroptosis via the inhibition of MAPK signaling pathways and predicts a good prognosis in sepsis. Cytokine 2020, 125, 154854. [Google Scholar] [CrossRef]
- Alsahli, M.A.; Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Anwar, S.; Almutary, A.G.; Alrumaihi, F.; Rahmani, A.H. 6-gingerol, a major ingredient of ginger attenuates diethylnitrosamine-induced liver injury in rats through the modulation of oxidative stress and anti-inflammatory activity. Mediat. Inflamm. 2021, 2021, 6661937. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, C.; Luo, J.; Hua, S.; Li, D.; Peng, L.; Liu, H.; Song, L. The protective role of Zingerone in a murine asthma model via activation of the AMPK/Nrf2/HO-1 pathway. Food Funct. 2021, 12, 3120–3131. [Google Scholar] [CrossRef]
- Lim, Y.J.; Min, H.Y.; Jang, W.G. Zingerone attenuates pi-induced vascular calcification via AMPK-mediated TIMP4 expression. J. Lipid Atheroscler. 2021, 10, 62–73. [Google Scholar] [CrossRef]
- Danwilai, K.; Konmun, J.; Sripanidkulchai, B.O.; Subongkot, S. Antioxidant activity of ginger extract as a daily supplement in cancer patients receiving adjuvant chemotherapy: A pilot study. Cancer Manag. Res. 2017, 9, 11–18. [Google Scholar] [CrossRef]
- Chen, H.; Fu, J.; Chen, H.; Hu, Y.; Soroka, D.N.; Prigge, J.R.; Schmidt, E.E.; Yan, F.; Major, M.B.; Chen, X.; et al. Ginger compound [6]-shogaol and its cysteine-conjugated metabolite (M2) activate Nrf2 in colon epithelial cells in vitro and in vivo. Chem. Res. Toxicol. 2014, 27, 1575–1585. [Google Scholar] [CrossRef]
- Ji, K.; Fang, L.; Zhao, H.; Li, Q.; Shi, Y.; Xu, C.; Wang, Y.; Du, L.; Wang, J.; Liu, Q. Ginger oleoresin alleviated γ-ray irradiation-induced reactive oxygen species via the nrf2 protective response in human mesenchymal stem cells. Oxidative Med. Cell. Longev. 2017, 2017, 1480294. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, A.; Bahrampour Juybari, K.; Fatemi, M.J.; Kamarul, T.; Bagheri, A.; Tekiyehmaroof, N.; Sharifi, A.M. Protective effect of ginger (Zingiber officinale Roscoe) extract against oxidative stress and mitochondrial apoptosis induced by interleukin-1β in cultured chondrocytes. Cells Tissues Organs 2017, 204, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Romero, A.; Forero, M.; Sequeda-Castañeda, L.G.; Grismaldo, A.; Iglesias, J.; Celis-Zambrano, C.A.; Schuler, I.; Morales, L. Effect of ginger extract on membrane potential changes and AKT activation on a peroxide-induced oxidative stress cell model. J. King Saud Univ.-Sci. 2018, 30, 263–269. [Google Scholar] [CrossRef]
- Elshopakey, G.E.; Almeer, R.; Alfaraj, S.; Albasher, G.; Abdelgawad, M.E.; Moneim, A.E.A.; Essawy, E.A. Zingerone mitigates inflammation, apoptosis and oxidative injuries associated with renal impairment in adriamycin-intoxicated mice. Toxin Rev. 2021, 41, 1–12. [Google Scholar] [CrossRef]
- Song, S.; Dang, M.; Kumar, M. Anti-inflammatory and renal protective effect of gingerol in high-fat diet/streptozotocin-induced diabetic rats via inflammatory mechanism. Inflammopharmacology 2019, 27, 1243–1254. [Google Scholar] [CrossRef]
- Rodrigues, F.A.; Prata, M.M.; Oliveira, I.C.; Alves, N.T.; Freitas, R.E.; Monteiro, H.S.; Silva, J.A.; Vieira, P.C.; Viana, D.A.; Libório, A.B.; et al. Gingerol fraction from Zingiber officinale protects against gentamicin-induced nephrotoxicity. Antimicrob. Agents Chemother. 2014, 58, 1872–1878. [Google Scholar] [CrossRef]
- El-Akabawy, G.; El-Kholy, W. Neuroprotective effect of ginger in the brain of streptozotocin-induced diabetic rats. Ann. Anat.-Anat. Anz. 2014, 196, 119–128. [Google Scholar] [CrossRef]
- Zhao, D.; Gu, M.Y.; Xu, J.L.; Zhang, L.J.; Ryu, S.Y.; Yang, H.O. Anti-neuroinflammatory effects of 12-dehydrogingerdione in LPS-activated microglia through inhibiting AKT/IKK/NF-κb pathway and activating Nrf-2/HO-1 pathway. Biomol. Ther. 2019, 27, 92–100. [Google Scholar] [CrossRef]
- Sapkota, A.; Park, S.J.; Choi, J.W. Neuroprotective effects of 6-shogaol and its metabolite, 6-paradol, in a mouse model of multiple sclerosis. Biomol. Ther. 2019, 27, 152–159. [Google Scholar] [CrossRef]
- Sistani Karampour, N.; Arzi, A.; Rezaie, A.; Pashmforoosh, M.; Kordi, F. Gastroprotective effect of Zingerone on ethanol-induced gastric ulcers in rats. Medicina 2019, 55, 64. [Google Scholar] [CrossRef]
- Saiah, W.; Halzoune, H.; Djaziri, R.; Tabani, K.; Koceir, E.A.; Omari, N. Antioxidant and gastroprotective actions of butanol fraction of Zingiber officinale against diclofenac sodium-induced gastric damage in rats. J. Food Biochem. 2018, 42, e12456. [Google Scholar] [CrossRef]
- Huang, H.C.; Chou, Y.C.; Wu, C.Y.; Chang, T.M. [8]-Gingerol inhibits melanogenesis in murine melanoma cells through down-regulation of the MAPK and PKA signal pathways. Biochem. Biophys. Res. Commun. 2013, 438, 375–381. [Google Scholar] [CrossRef]
- Wang, L.X.; Qian, J.; Zhao, L.N.; Zhao, S.H. Effects of volatile oil from ginger on the murine B16 melanoma cells and its mechanism. Food Funct. 2018, 9, 1058–1069. [Google Scholar] [CrossRef]
- Donkor, Y.O.; Abaidoo, C.S.; Tetteh, J.; Darko, N.D.; Atuahene, O.O.D.; Appiah, A.K.; Diby, T.; Maalman, R.S.E. The effect of Zingiber officinale (ginger) root ethanolic extract on the semen characteristics of adult male wistar rats. Int. J. Anat. Res. 2018, 6, 5481–5487. [Google Scholar] [CrossRef]
- Marak, N.R.; Malemnganbi, C.C.; Marak, C.R.; Mishra, L.K. Functional and antioxidant properties of cookies incorporated with foxtail millet and ginger powder. J. Food Sci. Technol. 2019, 56, 5087–5096. [Google Scholar] [CrossRef]
- Indiarto, R.; Subroto, E.; Angeline; Selly. Ginger rhizomes (Zingiber officinale) functionality in food and health perspective: A review. Food Res. 2021, 5, 497–505. [Google Scholar] [CrossRef]
- Anita, N.; Alam, G. Ginger candy (Zingiber officinale) reduces the frequency of vomiting of first-trimester pregnant women with emesis gravidarum. Enferm. Clín. 2020, 30, 536–538. [Google Scholar] [CrossRef]
- Fortune Business Insights. Ginger Tea Market Size, Share & Industry Analysis, Forecast 2022–2029. 2021. Available online: https://www.fortunebusinessinsights.com/ginger-tea-market-106069 (accessed on 15 March 2022).
- Smith, T.; Majid, F.; Eckl, V.; Reynolds, C.M. Herbal supplement sales in US increase by record-breaking 17.3% in 2020. HerbalGram 2021, 131, 52–65. Available online: http://herbalgram.org/resources/herbalgram/issues/131/table-of-contents/hg131-mkrpt/ (accessed on 2 September 2022).
- Weidner, M.S.; Sigwart, K. The safety of a ginger extract in the rat. J. Ethnopharmacol. 2000, 73, 513–520. [Google Scholar] [CrossRef]
- Rong, X.; Peng, G.; Suzuki, T.; Yang, Q.; Yamahara, J.; Li, Y. A 35-day gavage safety assessment of ginger in rats. Regul. Toxicol. Pharmacol. 2009, 54, 118–123. [Google Scholar] [CrossRef]
- Benny, M.; Shylaja, M.R.; Antony, B.; Gupta, N.K.; Mary, R.; Anto, A.; Jacob, S. Acute and sub acute toxicity studies with ginger extract in rats. Int. J. Pharm. Sci. Res. 2021, 12, 2799. [Google Scholar] [CrossRef]
- Jeena, K.; Liju, V.B.; Kuttan, R. A Preliminary 13-Week Oral Toxicity Study of Ginger Oil in Male and Female Wistar Rats. Int. J. Toxicol. 2011, 30, 662–670. [Google Scholar] [CrossRef]
- Idang, E.O.; Yemitan, O.; Ogbuagu, E.O.; Yemitan, O.K.; Mbagwu, H.O.C.; Udom, G.J.; Udobang, J.A. Toxicological assessment of Zingiber officinale Roscoe (ginger) root oil extracts in albino rats. Toxicol. Dig. 2019, 4, 108–119. [Google Scholar]
- Weidner, M.S.; Sigwart, K. Investigation of the teratogenic potential of a zingiber officinale extract in the rat. Reprod. Toxicol. 2001, 15, 75–80. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Effect of ginger tea on the fetal development of Sprague-Dawley rats. Reprod. Toxicol. 2000, 14, 507–512. [Google Scholar] [CrossRef]
- Stanisiere, J.; Mousset, P.Y.; Lafay, S. How safe is ginger rhizome for decreasing nausea and vomiting in women during early pregnancy? Foods 2018, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Ryan, J.L.; Morrow, G.R. Ginger. In Oncol. Nurse Ed.; 2010; 24, pp. 46–49. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5008850/ (accessed on 2 September 2022).
- Modi, M.; Modi, K. Ginger Root. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK565886/ (accessed on 15 March 2022).
- Schmidt, J.; Dahl, S.; Sherson, D.L. Allergic rhinoconjunctivitis caused by occupational exposure to ginger. Ugeskr Laeger 2015, 177, V12140723. [Google Scholar] [PubMed]
- Cueva, B.; Izquierdo, G.; Crespo, J.F.; Rodriguez, J. Unexpected spice allergy in the meat industry. J. Allergy Clin. Immunol. 2001, 108, 144. [Google Scholar] [CrossRef]
- van Toorenenbergen, A.W.; Dieges, P.H. lmmunoglobulin E antibodies against coriander and other spices. J. Allergy Clin. Immunol. 1985, 76, 477–481. [Google Scholar] [CrossRef]
- Gehlhaar, P.; de Olano, D.G.; Madrigal-Burgaleta, R.; Bartolomé, B.; Pastor-Vargas, C. Allergy to ginger with cysteine proteinase GP-I as the relevant allergen. Ann. Allergy Asthma Immunol. 2018, 121, 624–625. [Google Scholar] [CrossRef] [PubMed]
- Kanerva, L.; Estlander, T.; Jolanki, R. Occupational allergic contact dermatitis from spices. Contact Dermat. 1996, 35, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Kim, S.Y.; Yoo, H.H. Effects of an aqueous-ethanolic extract of ginger on cytochrome P450 enzyme-mediated drug metabolism. Die Pharm. 2012, 67, 1007–1009. [Google Scholar]
- Qiu, J.X.; Zhou, Z.W.; He, Z.X.; Zhang, X.; Zhou, S.F.; Zhu, S. Estimation of the binding modes with important human cytochrome P450 enzymes, drug interaction potential, pharmacokinetics, and hepatotoxicity of ginger components using molecular docking, computational, and pharmacokinetic modeling studies. Drug Des. Dev. Ther. 2015, 9, 841–866. [Google Scholar] [CrossRef]
- Gressenberger, P.; Rief, P.; Jud, P.; Gütl, K.; Muster, V.; Ghanim, L.; Brodmann, M.; Gary, T. Increased bleeding risk in a patient with oral anticoagulant therapy and concomitant herbal intake—A case report. eJIFCC 2019, 30, 95–98. [Google Scholar]
- Rubin, D.; Patel, V.; Dietrich, E. Effects of oral ginger supplementation on the INR. Case Rep. Med. 2019, 2019, 8784029. [Google Scholar] [CrossRef]
- Lesho, E.P.; Saullo, L.; Udvari-Nagy, S. A 76-year-old woman with erratic anticoagulation. Clevel. Clin. J. Med. 2004, 71, 651–656. [Google Scholar] [CrossRef]
- Krüth, P.; Brosi, E.; Fux, R.; Mörike, K.; Gleiter, C.H. Ginger-associated overanticoagulation by phenprocoumon. Ann. Pharmacother. 2004, 38, 257–260. [Google Scholar] [CrossRef]
- Shalansky, S.; Lynd, L.; Richardson, K.; Ingaszewski, A.; Kerr, C. Risk of Warfarin-related bleeding events and supratherapeutic International Normalized Ratios associated with complementary and alternative medicine: A longitudinal analysis. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2007, 27, 1237–1247. [Google Scholar] [CrossRef]
- Jiang, X.; Williams, K.M.; Liauw, W.S.; Ammit, A.J.; Roufogalis, B.D.; Duke, C.C.; Day, R.O.; McLachlan, A.J. Effect of ginkgo and ginger on the pharmacokinetics and pharmacodynamics of warfarin in healthy subjects. Br. J. Clin. Pharmacol. 2005, 59, 425–432. [Google Scholar] [CrossRef]
- Marx, W.; McKavanagh, D.; McCarthy, A.L.; Bird, R.; Ried, K.; Chan, A.; Isenring, L. The effect of ginger (Zingiber officinale) on platelet aggregation: A systematic literature review. PLoS ONE 2015, 10, e0141119. [Google Scholar] [CrossRef]
- Committee on Herbal Medicinal Products. Assessment Report on Zingiber Officinale Roscoe, Rhizoma; Technical Report EMA/HMPC/577856/2010; European Medicines Agency: London, UK, 2012; Available online: https://www.ema.europa.eu/en/documents/herbal-report/final-assessment-report-zingiber-officinale-roscoe-rhizoma_en.pdf (accessed on 2 September 2022).
- Revol, B.; Gautier-Veyret, E.; Arrivé, C.; Fouilhé Sam-Laï, N.; McLeer-Florin, A.; Pluchart, H.; Pinsolle, J.; Toffart, A. Pharmacokinetic herb-drug interaction between ginger and crizotinib. Br. J. Clin. Pharmacol. 2020, 86, 1892–1893. [Google Scholar] [CrossRef]
- Chiang, H.M.; Chao, P.D.L.; Hsiu, S.L.; Wen, K.C.; Tsai, S.Y.; Hou, Y.C. Ginger significantly decreased the oral bioavailability of cyclosporine in rats. Am. J. Chin. Med. 2006, 34, 845–855. [Google Scholar] [CrossRef]
- Okonta, J.; Uboh, M.; Obonga, W. Herb-drug interaction: A case study of effect of ginger on the pharmacokinetic of metronidazole in rabbit. Indian J. Pharm. Sci. 2008, 70, 230–232. [Google Scholar] [CrossRef]
- Mukkavilli, R.; Yang, C.; Singh Tanwar, R.; Ghareeb, A.; Luthra, L.; Aneja, R. Absorption, metabolic stability, and pharmacokinetics of ginger phytochemicals. Molecules 2017, 22, 553. [Google Scholar] [CrossRef]
- Yiannikourides, A.; Latunde-Dada, G. A short review of iron metabolism and pathophysiology of iron disorders. Medicines 2019, 6, 85. [Google Scholar] [CrossRef]
- Wallace, D.F. The regulation of iron absorption and homeostasis. Clin. Biochem. Rev. 2016, 37, 51–62. [Google Scholar]
- Tandara, L.; Salamunic, I. Iron metabolism: Current facts and future directions. Biochem. Med. 2012, 22, 311–328. [Google Scholar] [CrossRef]
- Dev, S.; Babitt, J.L. Overview of iron metabolism in health and disease. Hemodial. Int. 2017, 21, S6–S20. [Google Scholar] [CrossRef]
- Anderson, G.J.; Frazer, D.M. Current understanding of iron homeostasis. Am. J. Clin. Nutr. 2017, 106, 1559S–1566S. [Google Scholar] [CrossRef] [Green Version]
- West, A.R.; Oates, P.S. Mechanisms of heme iron absorption: Current questions and controversies. World J. Gastroenterol. 2008, 14, 4101–4110. [Google Scholar] [CrossRef]
- Camaschella, C.; Nai, A.; Silvestri, L. Iron metabolism and iron disorders revisited in the hepcidin era. Haematologica 2020, 105, 260–272. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Jimenez, K.; Kulnigg-Dabsch, S.; Gasche, C. Management of iron deficiency anemia. In Gastroenterol. Hepatol.; 2015; 11, pp. 241–250. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4836595/ (accessed on 2 September 2022).
- Elstrott, B.; Khan, L.; Olson, S.; Raghunathan, V.; DeLoughery, T.; Shatzel, J.J. The role of iron repletion in adult iron deficiency anemia and other diseases. Eur. J. Haematol. 2020, 104, 153–161. [Google Scholar] [CrossRef]
- Wieczorek, M.; Schwarz, F.; Sadlon, A.; Abderhalden, L.A.; de Godoi Rezende Costa Molino, C.; Spahn, D.R.; Schaer, D.J.; Orav, E.J.; Egli, A.; Bischoff-Ferrari, H.A. Iron deficiency and biomarkers of inflammation: A 3-year prospective analysis of the DO-HEALTH trial. Aging Clin. Exp. Res. 2022, 34, 515–525. [Google Scholar] [CrossRef]
- Weiss, G.; Ganz, T.; Goodnough, L.T. Anemia of inflammation. Blood 2019, 133, 40–50. [Google Scholar] [CrossRef]
- Pasricha, S.R.S.; Tye-Din, J.; Muckenthaler, M.U.; Swinkels, D.W. Iron deficiency. Lancet 2021, 397, 233–248. [Google Scholar] [CrossRef]
- Short, M.W.; Domagalski, J.E. Iron deficiency anemia: Evaluation and management. Am. Fam. Physician 2013, 87, 98–104. Available online: https://www.aafp.org/pubs/afp/issues/2013/0115/p98.html (accessed on 2 September 2022).
- Casgrain, A.; Collings, R.; Harvey, L.J.; Hooper, L.; Fairweather-Tait, S.J. Effect of iron intake on iron status: A systematic review and meta-analysis of randomized controlled trials. Am. J. Cinical Nutr. 2012, 96, 768–780. [Google Scholar] [CrossRef]
- Neto, L.G.R.S.; dos Santos Neto, J.E.; Bueno, N.B.; de Oliveira, S.L.; da Rocha Ataide, T. Effects of iron supplementation versus dietary iron on the nutritional iron status: Systematic review with meta-analysis of randomized controlled trials. Crit. Rev. Food Sci. Nutr. 2019, 59, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Houston, B.L.; Hurrie, D.; Graham, J.; Perija, B.; Rimmer, E.; Rabbani, R.; Bernstein, C.N.; Turgeon, A.F.; Fergusson, D.A.; Houston, D.S.; et al. Efficacy of iron supplementation on fatigue and physical capacity in non-anaemic iron-deficient adults: A systematic review of randomised controlled trials. BMJ Open 2018, 8, e019240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Blood Authority Australia. Iron Product Choice and Dose Calculation for Adults: Guidance for Australian Health Providers (March 2016); National Blood Authority: Canberra, ACT, Australia, 2016. [Google Scholar]
- Pasricha, S.R.S.; Flecknoe-Brown, S.C.; Allen, K.J.; Gibson, P.R.; McMahon, L.P.; Olynyk, J.K.; Roger, S.D.; Savoia, H.F.; Tampi, R.; Thomson, A.R.; et al. Diagnosis and management of iron deficiency anaemia: A clinical update. Med. J. Aust. 2010, 193, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Scarcello, E.; Herpain, A.; Tomatis, M.; Turci, F.; Jacques, P.J.; Lison, D. Hydroxyl radicals and oxidative stress: The dark side of Fe corrosion. Colloids Surf. B Biointerfaces 2020, 185, 110542. [Google Scholar] [CrossRef]
- Qi, X.; Zhang, Y.; Guo, H.; Hai, Y.; Luo, Y.; Yue, T. Mechanism and intervention measures of iron side effects on the intestine. Crit. Rev. Food Sci. Nutr. 2020, 60, 2113–2125. [Google Scholar] [CrossRef]
- Tiwari, A.K.M.; Mahdi, A.A.; Chandyan, S.; Zahra, F.; Godbole, M.M.; Jaiswar, S.P.; Srivastava, V.K.; Negi, M.P.S. Oral iron supplementation leads to oxidative imbalance in anemic women: A prospective study. Clin. Nutr. 2011, 30, 188–193. [Google Scholar] [CrossRef]
- Yilmaz, B.; Li, H. Gut microbiota and iron: The crucial actors in health and disease. Pharmaceuticals 2018, 11, 98. [Google Scholar] [CrossRef]
- Paganini, D.; Zimmermann, M.B. The effects of iron fortification and supplementation on the gut microbiome and diarrhea in infants and children: A review. Am. J. Clin. Nutr. 2017, 106, 1688S–1693S. [Google Scholar] [CrossRef]
- Rusu, I.G.; Suharoschi, R.; Vodnar, D.C.; Pop, C.R.; Socaci, S.A.; Vulturar, R.; Istrati, M.; Moroșan, I.; Fărcaș, A.C.; Kerezsi, A.D.; et al. Iron supplementation influence on the gut microbiota and probiotic intake effect in iron deficiency—A literature-based review. Nutrients 2020, 12, 1993. [Google Scholar] [CrossRef]
- Botta, A.; Barra, N.G.; Lam, N.H.; Chow, S.; Pantopoulos, K.; Schertzer, J.D.; Sweeney, G. Iron reshapes the gut microbiome and host metabolism. J. Lipid Atheroscler. 2021, 10, 160–183. [Google Scholar] [CrossRef]
- Baumgartner, J.; Smuts, C.M.; Aeberli, I.; Malan, L.; Tjalsma, H.; Zimmermann, M.B. Overweight impairs efficacy of iron supplementation in iron-deficient South African children: A randomized controlled intervention. Int. J. Obes. 2013, 37, 24–30. [Google Scholar] [CrossRef]
- Htet, M.K.; Fahmida, U.; Dillon, D.; Akib, A.; Utomo, B.; Thurnham, D.I. Is iron supplementation influenced by sub-clinical inflammation?: A randomized controlled trial among adolescent schoolgirls in Myanmar. Nutrients 2019, 11, 918. [Google Scholar] [CrossRef] [Green Version]
- Yuen, H.W.; Becker, W. Iron toxicity. In StatPearls; StatPearls Publishing: Tampa, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK459224/ (accessed on 15 March 2022).
- Abhilash, K.P.P.; Arul, J.J.; Bala, D. Fatal overdose of iron tablets in adults. Indian J. Crit. Care Med. 2013, 17, 311–313. [Google Scholar] [CrossRef]
- Barton, J.C.; Lee, P.L.; West, C.; Bottomley, S.S. Iron overload and prolonged ingestion of iron supplements: Clinical features and mutation analysis of hemochromatosis-associated genes in four cases. Am. J. Hematol. 2006, 81, 760–767. [Google Scholar] [CrossRef]
- Prakash, U.N.S.; Srinivasan, K. Enhanced intestinal uptake of iron, zinc and calcium in rats fed pungent spice principles—Piperine, capsaicin and ginger (Zingiber officinale). J. Trace Elem. Med. Biol. 2013, 27, 184–190. [Google Scholar] [CrossRef]
- Jaiswal, A.; Pathania, V.; A, J.L. An exploratory trial of food formulations with enhanced bioaccessibility of iron and zinc aided by spices. LWT 2021, 143, 111122. [Google Scholar] [CrossRef]
- Kulkarni, R.A.; Deshpande, A.; Saxena, K.; Varma, M.; Sinha, A.R. Ginger supplementary therapy for iron absorption in iron deficiency anemia. Indian J. Tradit. Knowl. 2012, 11, 78–80. [Google Scholar]
- Kulkarni, R.A. A Study of Anti-Inflammatory and Antioxidant Effects of Zingiber Officinale in Tuberculosis Patients with Anemia. Ph.D. Thesis, Shri Aurobindo Institute of Medical Sciences, Indore, MP, India, 2010. [Google Scholar]
- Prakash, U.N.S.; Srinivasan, K. Gastrointestinal protective effect of dietary spices during ethanol-induced oxidant stress in experimental rats. Appl. Physiol. Nutr. Metab. 2010, 35, 134–141. [Google Scholar] [CrossRef]
- Masuda, Y.; Kikuzaki, H.; Hisamoto, M.; Nakatani, N. Antioxidant properties of gingerol related compounds from ginger. BioFactors 2004, 21, 293–296. [Google Scholar] [CrossRef]
- Oboh, G.; Akinyemi, A.J.; Ademiluyi, A.O. Antioxidant and inhibitory effect of red ginger (Zingiber officinale var. Rubra) and white ginger (Zingiber officinale Roscoe) on Fe2+ induced lipid peroxidation in rat brain in vitro. Exp. Toxicol. Pathol. 2012, 64, 31–36. [Google Scholar] [CrossRef]
- Hinneburg, I.; Dorman, H.J.D.; Hiltunen, R. Antioxidant activities of extracts from selected culinary herbs and spices. Food Chem. 2006, 97, 122–129. [Google Scholar] [CrossRef]
- Kumar, S.; Saxena, K.; Uday, I.; Singh, N.; Saxena, R.; Singh, U.N. Anti-inflammatory action of ginger: A critical review in anemia of inflammation and its future aspects. Int. J. Herb. Med. 2013, 1, 16–20. Available online: https://www.florajournal.com/archives/2013/vol1issue4/PartA/2.1.pdf (accessed on 2 September 2022).
- Wang, J.; Chen, Y.; Hu, X.; Feng, F.; Cai, L.; Chen, F. Assessing the effects of ginger extract on polyphenol profiles and the subsequent impact on the fecal microbiota by simulating digestion and fermentation in vitro. Nutrients 2020, 12, 3194. [Google Scholar] [CrossRef]
- Wang, J.; Wang, P.; Li, D.; Hu, X.; Chen, F. Beneficial effects of ginger on prevention of obesity through modulation of gut microbiota in mice. Eur. J. Nutr. 2020, 59, 699–718. [Google Scholar] [CrossRef]
- Ma, Z.J.; Wang, H.J.; Ma, X.J.; Li, Y.; Yang, H.J.; Li, H.; Su, J.R.; Zhang, C.E.; Huang, L.Q. Modulation of gut microbiota and intestinal barrier function during alleviation of antibiotic-associated diarrhea with Rhizoma: Zingiber officinale (Ginger) extract. Food Funct. 2020, 11, 10839–10851. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, D.; Jiang, H.; Zhang, S.; Pang, X.; Gao, S.; Zhang, H.; Zhang, S.; Xiao, Q.; Chen, L.; et al. Gut microbiota variation with short-term intake of ginger juice on human health. Front. Microbiol. 2021, 11, 576061. [Google Scholar] [CrossRef]
- Ferri-Lagneau, K.F.; Moshal, K.S.; Grimes, M.; Zahora, B.; Lv, L.; Sang, S.; Leung, T. Ginger stimulates hematopoiesis via Bmp pathway in zebrafish. PLoS ONE 2012, 7, e39327. [Google Scholar] [CrossRef]
- Ferri-Lagneau, K.F.; Haider, J.; Sang, S.; Leung, T. Rescue of hematopoietic stem/progenitor cells formation in plcg1 zebrafish mutant. Sci. Rep. 2019, 9, 244. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, M.; Woloshun, R.R.; Yu, Y.; Lee, J.K.; Flores, S.R.; Merlin, D.; Collins, J.F. Oral administration of ginger-derived lipid nanoparticles and dmt1 sirna potentiates the effect of dietary iron restriction and mitigates pre-existing iron overload in hamp ko mice. Nutrients 2021, 13, 1686. [Google Scholar] [CrossRef]
- Gholampour, F.; Ghiasabadi, F.B.; Owji, S.M.; Vatanparast, J. The protective effect of hydroalcoholic extract of ginger (Zingiber officinale Rosc.) against iron-induced functional and histological damages in rat liver and kidney. In Avicenna J. Phytomed.; 2017; 7, pp. 542–553. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5745538/ (accessed on 2 September 2022).
- Golombick, T.; Diamond, T.H.; Manoharan, A.; Ramakrishna, R.; Badmaev, V. Effect of the ginger derivative, 6-shogaol, on ferritin levels in patients with low to intermediate-1-risk myelodysplastic syndrome-A small, investigative study. Clin. Med. Insights Blood Disord. 2017, 10, 1–4. [Google Scholar] [CrossRef] [PubMed]
- El-Refai, A.A.; Ghoniem, G.A.; El-Khateeb, A.Y.; Hassaan, M.M. Eco-friendly synthesis of metal nanoparticles using ginger and garlic extracts as biocompatible novel antioxidant and antimicrobial agents. J. Nanostruct. Chem. 2018, 8, 71–81. [Google Scholar] [CrossRef]
- Kirdat, P.N.; Dandge, P.B.; Hagwane, R.M.; Nikam, A.S.; Mahadik, S.P.; Jirange, S.T. Synthesis and characterization of ginger (z. officinale) extract mediated iron oxide nanoparticles and its antibacterial activity. Mater. Today Proc. 2020, 43, 2826–2831. [Google Scholar] [CrossRef]
- Noor, R.; Yasmin, H.; Ilyas, N.; Nosheen, A.; Hassan, M.N.; Mumtaz, S.; Khan, N.; Ahmad, A.; Ahmad, P. Comparative analysis of iron oxide nanoparticles synthesized from ginger (Zingiber officinale) and cumin seeds (Cuminum cyminum) to induce resistance in wheat against drought stress. Chemosphere 2022, 292, 133201. [Google Scholar] [CrossRef]
- Ooi, S.L.; Campbell, R.; Pak, S.C.; Golombick, T.; Manoharan, A.; Ramakrishna, R.; Badmaev, V.; Schloss, J. Is 6-shogaol an effective phytochemical for patients with lower-risk myelodysplastic syndrome? A narrative review. Integr. Cancer Ther. 2021, 20, 1–12. [Google Scholar] [CrossRef]
- Goodnough, L.T.; Nemeth, E.; Ganz, T. Detection, evaluation, and management of iron-restricted erythropoiesis. Blood 2010, 116, 4754–4761. [Google Scholar] [CrossRef]
- Huang, Q.; Feng, L.; Li, H.; Zheng, L.; Qi, X.; Wang, Y.; Feng, Q.; Liu, Z.; Liu, X.; Lu, L. Jian-Pi-Bu-Xue-Formula alleviates cyclophosphamide-induced myelosuppression via up-regulating NRF2/HO1/NQO1 signaling. Front. Pharmacol. 2020, 11, 1302. [Google Scholar] [CrossRef]
- Lam, C.T.W.; Chan, P.H.; Lee, P.S.C.; Lau, K.M.; Kong, A.Y.Y.; Gong, A.G.W.; Xu, M.L.; Lam, K.Y.C.; Dong, T.T.X.; Lin, H.; et al. Chemical and biological assessment of Jujube (Ziziphus jujuba)-containing herbal decoctions: Induction of erythropoietin expression in cultures. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1026, 254–262. [Google Scholar] [CrossRef]
- Chan, S.M.; Nelson, E.A.; Leung, S.S.; Cheung, P.C.; Li, C.Y. Special postpartum dietary practices of Hong Kong Chinese women. Eur. J. Clin. Nutr. 2000, 54, 797–802. [Google Scholar] [CrossRef]
- Zhang, L.; Gu, F.X.; Chan, J.M.; Wang, A.Z.; Langer, R.S.; Farokhzad, O.C. Nanoparticles in medicine: Therapeutic applications and developments. Clin. Pharmacol. Ther. 2008, 83, 761–769. [Google Scholar] [CrossRef]
- Zdrojewicz, Z.; Waracki, M.; Bugaj, B.; Pypno, D.; Cabała, K. Medical applications of nanotechnology. Adv. Hyg. Exp. Med. 2015, 69, 1196–1204. [Google Scholar] [CrossRef]
- Saha, P.K.; Saha, L. Iron nanoparticles and its potential application: A literature review. Indian J. Pharmacol. 2021, 53, 339–340. [Google Scholar] [CrossRef]
- Kumari, A.; Chauhan, A.K. Iron nanoparticles as a promising compound for food fortification in iron deficiency anemia: A review. J. Food Sci. Technol. 2021, 59, 1–17. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]


| Nutrient | Amount | Unit |
|---|---|---|
| Carbohydrate | 39.70–58.21 | % |
| Protein | 11.65–12.05 | % |
| Crude fibre | 8.30–21.90 | % |
| Fat | 9.89–17.11 | % |
| Moisture | 3.95–4.63 | % |
| Ash | 4.95–7.45 | % |
| -carotene | 0.68–0.81 | mg/100 g |
| Ascorbic acid | 2.2–3.8 | mg/100 g |
| Polyphenols | 11.8–12.5 | mg/100 g |
| Calcium | 64.4–69.2 | mg/100 g |
| Iron | 1.5–1.8 | mg/100 g |
| Copper | 0.46–0.75 | mg/100 g |
| Beneficial Property | Study Type | Research Findings | Reference |
|---|---|---|---|
| Iron absorption enhancement | Ex vivo | Ginger was the most potent spice for enhancing iron absorption by increasing uptake by 28.5 ± 2.09% in the jejunum of rats compared to control. | [137] |
| In vitro | Adding ginger to food enhanced the bioaccessibility of dietary iron by 2- to 3-fold depending on the formulations. | [138] | |
| Human study | Ginger plus oral iron therapy improved haematological and iron parameters of anaemic patients better than oral iron therapy alone. | [139,140] | |
| Antioxidant activity | In vivo | Adding ginger to the diet significantly increased the activities of antioxidant enzymes () at the intestinal and gastric mucosa of rats, demonstrating enhanced protective effects against oxidative stress. | [141] |
| In vitro | The polyphenols and diarylheptanoid derivatives of ginger contributed to both radical scavenging and inhibitory effects of autoxidation. | [142] | |
| In vitro | Both red and white ginger variants possessed antioxidant capacities against free iron radicals in rat brains, but red ginger was superior at inhibiting Fe2-induced lipid peroxidation and chelating Fe2. | [143] | |
| In vitro | Water-based extract of ginger showed relatively low antioxidant activities compared to other spices due to reduced phenolic contents produced from hydro-distillation extraction. | [144] | |
| Anti-inflammatory action | Review | The bioactive compounds in ginger possessed broad anti-inflammatory properties that can block the activation of NF- by suppressing pro-inflammatory cytokines of IL-1, TNF- and IL-6, thus preventing hepcidin production. | [145] |
| Human study | Ginger plus oral iron therapy significantly reduced the inflammatory marker TNF- () in anaemic patients better than oral iron therapy alone. | [139,140] | |
| Gut microbiota modulation | In vitro | Undigested ginger polyphenols significantly increased the abundances of Bifidobacterium () and Enterococcus () after faecal inoculated fermentation, accompanied by elevated levels of SCFA and decreased pH value. | [146] |
| In vivo | Ginger supplementation could mitigate the detrimental impact of a high-fat diet in mice by promoting the abundance of Bifidobacterium genus and SCFA-producing bacteria (Alloprevotella and Allobaculum). | [147] | |
| In vivo | Ginger treatment significantly reduced antibiotic-associated diarrhoea symptoms () in rats with an associated increase in microbiota diversity and improved intestinal barrier integrity. | [148] | |
| Human study | Ginger juice consumption in healthy adults decreased the Prevotella-to-Bacteroides ratio and pro-inflammatory Ruminococcus_1 and Ruminococcus_2 genus while increasing the Firmicutes-to-Bacteroidetes ratio, Proteobacteria and anti-inflammatory Faecalibacterium. | [149] | |
| Erythropoiesis stimulation | In vivo | Ginger, with its bioactive compounds of 8-gingerol, 10-gingerol, 8-shogaol, and 10-shogaol, promoted the expression of Gata1 in erythroid cells of zebrafish embryos through the Bmp signalling pathway. | [150] |
| In vivo | Ginger induced scl/runx1 expression through Bmp and Notch signalling pathways which up-regulated nitric oxide production for regeneration of haematopoietic stem/progenitor cells. | [151] | |
| Iron overload prevention | In vivo | The bioactive lipids in ginger repressed some iron-related parameters, including reductions in 20% of 59Fe absorption, 65% of pancreatic non-haem iron, and 40% to 50% of serum ferritin levels, compared to controls. | [152] |
| In vivo | Ginger extract demonstrated strong protective effects against iron toxicity through its free radical scavenging activities in iron-overloaded rats. | [153] | |
| Case series | Ginger extract rich in 6-shogaol prevented iron overload in three patients with myelodysplastic syndrome. These patients had elevated serum ferritin (>300 g/L) at baseline but achieved >40% reductions after three months through upregulation of hepcidin. | [154] | |
| Ginger-synthesised iron nanoparticles | In vitro | Ginger was used to bio-reduce the metallic ions to nanoparticles (Fe3+ ions to FeNPs). Transmission electron microscopy showed that the FeNPs in ginger were in the range of 14.08–21.57 nm with almost spherical forms and demonstrated considerable radical scavenging properties and antimicrobial activities against Gram-positive and Gram-negative bacteria and fungi. | [155] |
| In vitro | Ginger can be a suitable green material for synthesising iron nanoparticles with high antioxidant and antibacterial properties. | [156,157] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ooi, S.L.; Pak, S.C.; Campbell, R.; Manoharan, A. Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism. Molecules 2022, 27, 6417. https://doi.org/10.3390/molecules27196417
Ooi SL, Pak SC, Campbell R, Manoharan A. Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism. Molecules. 2022; 27(19):6417. https://doi.org/10.3390/molecules27196417
Chicago/Turabian StyleOoi, Soo Liang, Sok Cheon Pak, Ron Campbell, and Arumugam Manoharan. 2022. "Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism" Molecules 27, no. 19: 6417. https://doi.org/10.3390/molecules27196417
APA StyleOoi, S. L., Pak, S. C., Campbell, R., & Manoharan, A. (2022). Polyphenol-Rich Ginger (Zingiber officinale) for Iron Deficiency Anaemia and Other Clinical Entities Associated with Altered Iron Metabolism. Molecules, 27(19), 6417. https://doi.org/10.3390/molecules27196417