Cold Sintering of Li6.4La3Zr1.4Ta0.6O12/PEO Composite Solid Electrolytes
Abstract
:1. Introduction
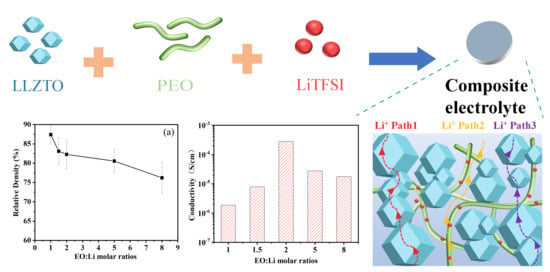
2. Results and Discussion
3. Materials and Methods
3.1. Materials Preparation
3.2. Cold Sintering Process
3.3. Sample Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Kousksou, T.; Bruel, P.; Jamil, A.; Rhafiki, T.E.; Zeraouli, Y. Energy storage: Applications and challenges. Sol. Energy Mater. Sol. Cells 2014, 120, 59–80. [Google Scholar] [CrossRef]
- Nitta, N.; Wu, F.; Lee, J.T.; Yushin, G. Li-ion battery materials: Present and future. Mater. Today 2015, 18, 252–264. [Google Scholar] [CrossRef]
- Tikekar, M.D.; Choudhury, S.; Tu, Z.; Archer, L.A. Design principles for electrolytes and interfaces for stable lithium-metal batteries. Nat. Energy 2016, 1, 1–7. [Google Scholar] [CrossRef]
- Qian, J.F.; Henderson, W.A.; Xu, W.; Bhattacharya, P.; Engelhard, M.; Borodin, O.; Zhang, J.G. High rate and stable cycling of lithium metal anode. Nat. Commun. 2015, 6, 6362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, F.; Xu, W.; Chen, X.; Zhang, J.; Engelhard, M.H.; Zhang, Y.; Johnson, B.R.; Crum, J.V.; Blake, T.A.; Liu, X. Effects of carbonate solvents and lithium salts on morphology and coulombic efficiency of lithium electrode. J. Electrochem. Soc. 2013, 160, A1894. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Kanno, R. Li10GeP2S12-type superionic conductors: Synthesis, structure, and ionic transportation. Adv. Energy Mater. 2020, 10, 2002153. [Google Scholar] [CrossRef]
- Kato, Y.; Hori, S.; Saito, T.; Suzuki, K.; Hirayama, M.; Mitsui, A.; Yonemura, M.; Iba, H.; Kanno, R. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy 2016, 1, 16030. [Google Scholar] [CrossRef]
- Manthiram, A.; Yu, X.; Wang, S. Lithium battery chemistries enabled by solid-state electrolytes. Nat. Rev. Mater. 2017, 2, 1–16. [Google Scholar] [CrossRef]
- Ohta, S.; Kobayashi, T.; Seki, J.; Asaoka, T. Electrochemical performance of an all-solid-state lithium ion battery with garnet-type oxide electrolyte. J. Power Sources 2012, 202, 332–335. [Google Scholar] [CrossRef]
- Liu, B.; Gong, Y.; Fu, K.; Han, X.; Yao, Y.; Pastel, G.; Yang, C.; Xie, H.; Wachsman, E.D.; Hu, L. Garnet solid electrolyte protected Li-metal batteries. ACS Appl. Mater. Interfaces 2017, 9, 18809–18815. [Google Scholar] [CrossRef]
- Murugan, R.; Thangadurai, V.; Weppner, W. Fast lithium ion conduction in garnet-type Li7La3Zr2O12. Angew. Chem. Int. Ed. 2007, 46, 7778–7781. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Wen, Z.; Wu, J.; Yang, X. Preparation and electrical properties of NASICON-type structured Li1.4Al0.4Ti1.6(PO4)3 glass-ceramics by the citric acid-assisted sol–gel method. Solid State Ion. 2007, 178, 29–34. [Google Scholar] [CrossRef]
- Morimoto, H.; Awano, H.; Terashima, J.; Shindo, Y.; Nakanishi, S.; Ito, N.; Ishikawa, K.; Tobishima, S.-I. Preparation of lithium ion conducting solid electrolyte of NASICON-type Li1+ xAlxTi2−x (PO4)3 (x = 0.3) obtained by using the mechanochemical method and its application as surface modification materials of LiCoO2 cathode for lithium cell. J. Power Sources 2013, 240, 636–643. [Google Scholar] [CrossRef]
- Kwon, W.J.; Kim, H.; Jung, K.-N.; Cho, W.; Kim, S.H.; Lee, J.-W.; Park, M.-S. Enhanced Li+ conduction in perovskite Li3xLa2/3− x☐1/3− 2xTiO3 solid-electrolytes via microstructural engineering. J. Mater. Chem. A 2017, 5, 6257–6262. [Google Scholar] [CrossRef]
- Jiang, Z.; Wang, S.; Chen, X.; Yang, W.; Yao, X.; Hu, X.; Han, Q.; Wang, H. Tape-casting Li0. 34La0. 56TiO3 ceramic electrolyte films permit high energy density of lithium-metal batteries. Adv. Mater. 2020, 32, 1906221. [Google Scholar] [CrossRef] [PubMed]
- Put, B.; Vereecken, P.M.; Meersschaut, J.; Sepúlveda, A.; Stesmans, A. Electrical characterization of ultrathin RF-sputtered LiPON layers for nanoscale batteries. ACS Appl. Mater. Interfaces 2016, 8, 7060–7069. [Google Scholar] [CrossRef]
- Zheng, F.; Kotobuki, M.; Song, S.; Lai, M.O.; Lu, L. Review on solid electrolytes for all-solid-state lithium-ion batteries. J. Power Sources 2018, 389, 198–213. [Google Scholar] [CrossRef]
- Gao, Z.; Sun, H.; Fu, L.; Ye, F.; Zhang, Y.; Luo, W.; Huang, Y. Promises, challenges, and recent progress of inorganic solid-state electrolytes for all-solid-state lithium batteries. Adv. Mater. 2018, 30, 1705702. [Google Scholar] [CrossRef]
- Kerman, K.; Luntz, A.; Viswanathan, V.; Chiang, Y.-M.; Chen, Z. Practical challenges hindering the development of solid state Li ion batteries. J. Electrochem. Soc. 2017, 164, A1731. [Google Scholar] [CrossRef]
- Nan, C.-W.; Fan, L.; Lin, Y.; Cai, Q. Enhanced ionic conductivity of polymer electrolytes containing nanocomposite SiO2 particles. Phys. Rev. Lett. 2003, 91, 266104. [Google Scholar] [CrossRef]
- Xia, Y.; Liang, Y.; Xie, D.; Wang, X.; Zhang, S.; Xia, X.; Gu, C.; Tu, J. A poly (vinylidene fluoride-hexafluoropropylene) based three-dimensional network gel polymer electrolyte for solid-state lithium-sulfur batteries. Chem. Eng. J. 2019, 358, 1047–1053. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Z.; Li, G.; Huang, H.; Xue, R.; Chen, L.; Wang, F. Lithium ion conduction in polymer electrolytes based on PAN. Solid State Ion. 1996, 85, 79–84. [Google Scholar] [CrossRef]
- Baskaran, R.; Selvasekarapandian, S.; Kuwata, N.; Kawamura, J.; Hattori, T. Conductivity and thermal studies of blend polymer electrolytes based on PVAc–PMMA. Solid State Ion. 2006, 177, 2679–2682. [Google Scholar] [CrossRef]
- Yan, J.; Miao, L.; Duan, H.; Zhu, D.; Lv, Y.; Li, L.; Gan, L.; Liu, M. High-energy aqueous supercapacitors enabled by N/O codoped carbon nanosheets and “water-in-salt” electrolyte. Chin. Chem. Lett. 2022, 33, 2681–2686. [Google Scholar] [CrossRef]
- Qin, Y.; Miao, L.; Mansuer, M.; Hu, C.; Lv, Y.; Gan, L.; Liu, M. Spatial confinement strategy for micelle-size-mediated modulation of mesopores in hierarchical porous carbon nanosheets with an efficient capacitive response. ACS Appl. Mater. Interfaces 2022, 14, 33328–33339. [Google Scholar] [CrossRef]
- Zhang, W.; Nie, J.; Li, F.; Wang, Z.L.; Sun, C. A durable and safe solid-state lithium battery with a hybrid electrolyte membrane. Nano Energy 2018, 45, 413–419. [Google Scholar] [CrossRef]
- Xie, H.; Yang, C.; Fu, K.; Yao, Y.; Jiang, F.; Hitz, E.; Liu, B.; Wang, S.; Hu, L. Flexible, scalable, and highly conductive garnet-polymer solid electrolyte templated by bacterial cellulose. Adv. Energy Mater. 2018, 8, 1703474. [Google Scholar] [CrossRef]
- Thangadurai, V.; Narayanan, S.; Pinzaru, D. Garnet-type solid-state fast Li ion conductors for Li batteries: Critical review. Chem. Soc. Rev. 2014, 43, 4714–4727. [Google Scholar] [CrossRef]
- Tong, X.; Thangadurai, V.; Wachsman, E.D. Highly conductive Li garnets by a multielement doping strategy. Inorg. Chem. 2015, 54, 3600–3607. [Google Scholar] [CrossRef]
- Choi, B.-K. Optical microscopy study on the crystallization in PEO-salt polymer electrolytes. Solid State Ion. 2004, 168, 123–129. [Google Scholar] [CrossRef]
- Fang, R.; Xu, B.; Grundish, N.S.; Xia, Y.; Li, Y.; Lu, C.; Liu, Y.; Wu, N.; Goodenough, J.B. Li2S6-integrated PEO-based polymer electrolytes for all-solid-state lithium-metal batteries. Angew. Chem. 2021, 133, 17842–17847. [Google Scholar] [CrossRef]
- Guo, J.; Zhao, X.; Herisson De Beauvoir, T.; Seo, J.H.; Berbano, S.S.; Baker, A.L.; Azina, C.; Randall, C.A. Recent progress in applications of the cold sintering process for ceramic–polymer composites. Adv. Funct. Mater. 2018, 28, 1801724. [Google Scholar] [CrossRef]
- Lee, W.; Lyon, C.K.; Seo, J.H.; Lopez-Hallman, R.; Leng, Y.; Wang, C.Y.; Hickner, M.A.; Randall, C.A.; Gomez, E.D. Ceramic–salt composite electrolytes from cold sintering. Adv. Funct. Mater. 2019, 29, 1807872. [Google Scholar] [CrossRef]
- Berbano, S.S.; Guo, J.; Guo, H.; Lanagan, M.T.; Randall, C.A. Cold sintering process of Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte. J. Am. Ceram. Soc. 2017, 100, 2123–2135. [Google Scholar] [CrossRef]
- Lim, H.-D.; Park, J.-H.; Shin, H.-J.; Jeong, J.; Kim, J.T.; Nam, K.-W.; Jung, H.-G.; Chung, K.Y. A review of challenges and issues concerning interfaces for all-solid-state batteries. Energy Storage Mater. 2020, 25, 224–250. [Google Scholar] [CrossRef]
- Guo, J.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Kupp, E.R.; Messing, G.L.; Randall, C.A. Cold sintering: A paradigm shift for processing and integration of ceramics. Angew. Chem. 2016, 128, 11629–11633. [Google Scholar] [CrossRef]
- Guo, J.; Berbano, S.S.; Guo, H.; Baker, A.L.; Lanagan, M.T.; Randall, C.A. Cold sintering process of composites: Bridging the processing temperature gap of ceramic and polymer materials. Adv. Funct. Mater. 2016, 26, 7115–7121. [Google Scholar] [CrossRef]
- Guo, H.; Guo, J.; Baker, A.; Randall, C.A. Hydrothermal-assisted cold sintering process: A new guidance for low-temperature ceramic sintering. ACS Appl. Mater. Interfaces 2016, 8, 20909–20915. [Google Scholar] [CrossRef]
- Maria, J.-P.; Kang, X.; Floyd, R.D.; Dickey, E.C.; Guo, H.; Guo, J.; Baker, A.; Funihashi, S.; Randall, C.A. Cold sintering: Current status and prospects. J. Mater. Res. 2017, 32, 3205–3218. [Google Scholar] [CrossRef] [Green Version]
- Wood, M.; Gao, X.; Shi, R.; Heo, T.W.; Espitia, J.A.; Duoss, E.B.; Wood, B.C.; Ye, J. Exploring the relationship between solvent-assisted ball milling, particle size, and sintering temperature in garnet-type solid electrolytes. J. Power Sources 2021, 484, 229252. [Google Scholar] [CrossRef]
- Thompson, T.; Wolfenstine, J.; Allen, J.L.; Johannes, M.; Huq, A.; David, I.N.; Sakamoto, J. Tetragonal vs. cubic phase stability in Al–free Ta doped Li7La3Zr2O12(LLZO). J. Mater. Chem. A 2014, 2, 13431–13436. [Google Scholar] [CrossRef]
- Huggins, R.A. Simple method to determine electronic and ionic components of the conductivity in mixed conductors a review. Ionics 2002, 8, 300–313. [Google Scholar] [CrossRef]
- Waidha, A.I.; Ferber, T.; Donzelli, M.; Hosseinpourkahvaz, N.; Vanita, V.; Dirnberger, K.; Ludwigs, S.; Hausbrand, R.; Jaegermann, W.; Clemens, O. Compositional Dependence of Li-Ion Conductivity in Garnet-Rich Composite Electrolytes for All-Solid-State Lithium-Ion Batteries—Toward Understanding the Drawbacks of Ceramic-Rich Composites. ACS Appl. Mater. Interfaces 2021, 13, 31111–31128. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, S.; Pan, Y.; Zhang, X.; Yan, Z.; Xiang, Y. Reduced Energy Barrier for Li+ Transport Across Grain Boundaries with Amorphous Domains in LLZO Thin Films. Nanoscale Res. Lett. 2020, 15, 153. [Google Scholar] [CrossRef] [PubMed]
- Irvine, J.T.; Sinclair, D.C.; West, A.R. Electroceramics: Characterization by impedance spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar]
- Wang, C.; Yang, Y.; Liu, X.; Zhong, H.; Xu, H.; Xu, Z.; Shao, H.; Ding, F. Suppression of lithium dendrite formation by using LAGP-PEO (LiTFSI) composite solid electrolyte and lithium metal anode modified by PEO (LiTFSI) in all-solid-state lithium batteries. ACS Appl. Mater. Interfaces 2017, 9, 13694–13702. [Google Scholar] [CrossRef]
- Svanberg, C.; Bergman, R.; Börjesson, L.; Jacobsson, P. Diffusion of solvent/salt and segmental relaxation in polymer gel electrolytes. Electrochim. Acta 2001, 46, 1447–1451. [Google Scholar] [CrossRef]
- Shen, F.; Guo, W.; Zeng, D.; Sun, Z.; Gao, J.; Li, J.; Zhao, B.; He, B.; Han, X. A Simple and Highly Efficient Method toward High-Density Garnet-Type LLZTO Solid-State Electrolyte. ACS Appl. Mater. Interfaces 2020, 12, 30313–30319. [Google Scholar] [CrossRef]
- Lei, C.; Shetty, D.K.; Simpson, M.F.; Virkar, A.V. Fabrication of high-density and translucent Al-containing garnet, Li7-xLa3Zr2-xTaxO12 (LLZTO) solid-state electrolyte by pressure filtration and sintering. Solid State Ion. 2021, 364, 115640. [Google Scholar] [CrossRef]
- Huang, X.; Liu, C.; Lu, Y.; Xiu, T.; Jin, J.; Badding, M.E.; Wen, Z. A Li-Garnet composite ceramic electrolyte and its solid-state Li-S battery. J. Power Sources 2018, 382, 190–197. [Google Scholar] [CrossRef]
- Froboese, L.; Groffmann, L.; Monsees, F.; Helmers, L.; Loellhoeffel, T.; Kwade, A. Enhancing the lithium ion conductivity of an all solid-state electrolyte via dry and solvent-free scalable series production processes. J. Electrochem. Soc. 2020, 167, 020558. [Google Scholar] [CrossRef]
- Hamao, N.; Kataoka, K.; Kijima, N.; Akimoto, J. Synthesis, crystal structure and conductive properties of garnet-type lithium ion conductor Al-free Li7−xLa3Zr2−xTaxO12 (0 ≤ x ≤ 0.6). J. Ceram. Soc. Jpn. 2016, 124, 678–683. [Google Scholar] [CrossRef] [Green Version]
- Hammons, J.A.; Espitia, J.A.; Ramos, E.; Shi, R.; Meisenkothen, F.; Wood, M.; Ceron, M.R.; Ye, J. Pore and grain chemistry during sintering of garnet-type Li6.4La3Zr1.4Ta0.6O12 solid-state electrolytes. J. Mater. Chem. A 2022, 10, 9080–9090. [Google Scholar] [CrossRef]
- Seo, J.-H.; Fan, Z.; Nakaya, H.; Rajagopalan, R.; Gomez, E.D.; Iwasaki, M.; Randall, C.A. Cold sintering, enabling a route to co-sinter an all-solid-state lithium-ion battery. Jpn. J. Appl. Phys. 2021, 60, 037001. [Google Scholar] [CrossRef]
- Wang, X.; Wang, J.; Li, F.; Zhu, F.; Ma, C. Influence of cold sintering process on the structure and properties of garnet-type solid electrolytes. Ceram. Int. 2020, 46, 18544–18550. [Google Scholar] [CrossRef]
- Lin, D.; Yuen, P.Y.; Liu, Y.; Liu, W.; Liu, N.; Dauskardt, R.H.; Cui, Y. A silica-aerogel-reinforced composite polymer electrolyte with high ionic conductivity and high modulus. Adv. Mater. 2018, 30, 1802661. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, J.; Shi, F.; Lin, D.; Liu, Y.; Liu, W.; Pei, A.; Gong, Y.; Wang, H.; Liu, K. Vertically aligned and continuous nanoscale ceramic–polymer interfaces in composite solid polymer electrolytes for enhanced ionic conductivity. Nano Lett. 2018, 18, 3829–3838. [Google Scholar] [CrossRef]
- Zhao, C.-Z.; Zhang, X.-Q.; Cheng, X.-B.; Zhang, R.; Xu, R.; Chen, P.-Y.; Peng, H.-J.; Huang, J.-Q.; Zhang, Q. An anion-immobilized composite electrolyte for dendrite-free lithium metal anodes. Proc. Natl. Acad. Sci. USA 2017, 114, 11069–11074. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Tang, M.; Hu, Y.Y. Lithium ion pathway within Li7La3Zr2O12-polyethylene oxide composite electrolytes. Angew. Chem. 2016, 128, 12726–12730. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, B.; Kang, S.; Zhao, X.; Zhang, J.; Wang, X.; Yang, Y.; Yang, L.; Liao, R. Cold Sintering of Li6.4La3Zr1.4Ta0.6O12/PEO Composite Solid Electrolytes. Molecules 2022, 27, 6756. https://doi.org/10.3390/molecules27196756
He B, Kang S, Zhao X, Zhang J, Wang X, Yang Y, Yang L, Liao R. Cold Sintering of Li6.4La3Zr1.4Ta0.6O12/PEO Composite Solid Electrolytes. Molecules. 2022; 27(19):6756. https://doi.org/10.3390/molecules27196756
Chicago/Turabian StyleHe, Binlang, Shenglin Kang, Xuetong Zhao, Jiexin Zhang, Xilin Wang, Yang Yang, Lijun Yang, and Ruijin Liao. 2022. "Cold Sintering of Li6.4La3Zr1.4Ta0.6O12/PEO Composite Solid Electrolytes" Molecules 27, no. 19: 6756. https://doi.org/10.3390/molecules27196756
APA StyleHe, B., Kang, S., Zhao, X., Zhang, J., Wang, X., Yang, Y., Yang, L., & Liao, R. (2022). Cold Sintering of Li6.4La3Zr1.4Ta0.6O12/PEO Composite Solid Electrolytes. Molecules, 27(19), 6756. https://doi.org/10.3390/molecules27196756