Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis
Abstract
:1. Introduction
2. Results
2.1. Antibacterial Activity against Clinical Isolates of S. aureus
2.2. Effect of CFCS on the Dynamic Growth of the S. aureus
2.3. Analysis of Antibacterial Active Substances in CFCS
2.4. Effects of Heat, pH and Metal Ions on Antibacterial Activity
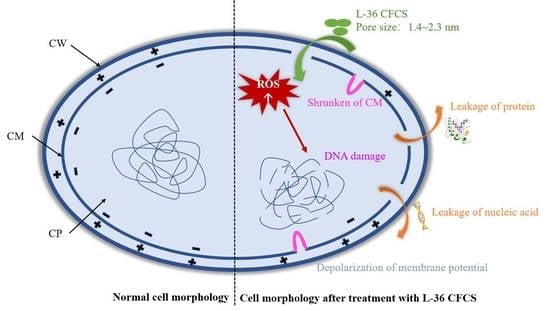
2.5. Mode of Action of L-36 CFCS
2.5.1. SEM and TEM
2.5.2. Effect of CFCS on Cell Membrane Integrity
2.5.3. Estimation of Pore Size in Cytomembrane of S. aureus
2.5.4. Effects of CFCS on Leakage of Nucleic Acid and Proteins in S. aureus
2.5.5. Effects of CFCS on Membrane Potential of S. aureus
2.5.6. Effects on Subcellular Structure of S. aureus by L-36 CFCS
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Culture Conditions
4.2. Antibacterial Activity
4.3. Minimal Inhibitory Concentration (MIC)
4.4. Effect of CFCS on the Dynamic Growth of the S. aureus
4.5. Analysis of Antibacterial Active Components of CFCS
4.6. Effects of Heat, pH, and Metal Ions on Antibacterial Activity
4.7. Mode of Action of L-36 CFCS
4.7.1. SEM and TEM
4.7.2. Effects on Cell Membranes of S. aureus by L-36 CFCS
4.7.3. Effects on Subcellular Structure of S. aureus by L-36 CFCS
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Das, D.; Panda, S.; Jena, B.; Sahoo, A. Economic Impact of Subclinical and Clinical Mastitis in Odisha, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 3651–3654. [Google Scholar] [CrossRef]
- Aghamohammadi, M.; Haine, D.; Kelton, D.F.; Barkema, H.; Hogeveen, H.; Keefe, G.P.; Dufour, S. Herd-Level Mastitis-Associated Costs on Canadian Dairy Farms. Front. Vet.-Sci. 2018, 5, 100. [Google Scholar] [CrossRef] [PubMed]
- Sharun, K.; Dhama, K.; Tiwari, R.; Gugjoo, M.B.; Iqbal Yatoo, M.; Patel, S.K.; Pathak, M.; Karthik, K.; Khurana, S.K.; Singh, R.; et al. Advances in therapeutic and managemental approaches of bovine mastitis: A comprehensive review. Vet. Quart. 2021, 41, 107–136. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Qureshi, S.; Kashoo, Z.; Farooq, S.; Wani, S.A.; Hussain, M.I.; Banday, M.S.; Khan, A.A.; Gull, B.; Habib, A.; et al. Methicillin resistance genes and in vitro biofilm formation among Staphylococcus aureus isolates from bovine mastitis in India. Comp. Immunol. Microbiol. Infect. Dis. 2019, 64, 117–124. [Google Scholar] [CrossRef]
- Siedler, S.; Balti, R.; Neves, A.R. Bioprotective mechanisms of lactic acid bacteria against fungal spoilage of food. Curr. Opin. Biotechnol. 2018, 56, 138–146. [Google Scholar] [CrossRef]
- Yeh, R.H.; Hsieh, C.W.; Chen, K.L. Screening lactic acid bacteria to manufacture two-stage fermented feed and pelleting to investigate the feeding effect on broilers. Poultry Sci. 2018, 97, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Gao, J.; He, X.; Gong, Z.; Wan, Y.; Hu, T.; Li, Y.; Cao, H. Lactobacillus rhamnosus GG-derived postbiotic prevents intestinal infection with enterohaemorrhagic E. coli O157: H7. Int. J. Infect. Dis. 2020, 101, 134. [Google Scholar] [CrossRef]
- Lizardo, R.C.M.; Cho, H.D.; Lee, J.H.; Won, Y.S.; Seo, K.I. Extracts of Elaeagnus multiflora Thunb. fruit fermented by lactic acid bacteria inhibit SW480 human colon adenocarcinoma via induction of cell cycle arrest and suppression of metastatic potential. J. Food Sci. 2020, 85, 2565–2577. [Google Scholar] [CrossRef]
- Sikorska, H.; Smoragiewicz, W. Role of probiotics in the prevention and treatment of meticillin-resistant Staphylococcus aureus infections. Int. J. Antimicrob. Agents 2013, 42, 475–481. [Google Scholar] [CrossRef]
- Karska-Wysocki, B.; Bazo, M.; Smoragiewicz, W. Antibacterial activity of Lactobacillus acidophilus and Lactobacillus casei against methicillin-resistant Staphylococcus aureus (MRSA). Microbiol. Res. 2010, 165, 674–686. [Google Scholar] [CrossRef]
- Olofsson, T.C.; Butler, È.; Markowicz, P.; Lindholm, C.; Larsson, L.; Vásquez, A. Lactic acid bacterial symbionts in honeybees–an unknown key to honey’s antimicrobial and therapeutic activities. Int. Wound J. 2016, 13, 668–679. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Zhang, X.; Xu, Z. Identification of antibacterial substances of Lactobacillus plantarum DY-6 for bacteriostatic action. Food Sci. Nutr. 2020, 8, 2854–2863. [Google Scholar] [CrossRef]
- Scillato, M.; Spitale, A.; Mongelli, G.; Privitera, G.F.; Mangano, K.; Cianci, A.; Stefani, S.; Santagati, M. Antimicrobial properties of Lactobacillus cell-free supernatants against multidrug-resistant urogenital pathogens. Microbiologyopen 2021, 10, e1173. [Google Scholar] [CrossRef]
- Dai, M.; Li, Y.; Xu, L.; Wu, D.; Zhou, Q.; Li, P.; Gu, Q. A Novel Bacteriocin from Lactobacillus Pentosus ZFM94 and Its Antibacterial Mode of Action. Front. Nutr. 2021, 8, 710862. [Google Scholar] [CrossRef] [PubMed]
- Wayah, S.B.; Philip, K. Pentocin MQ1: A novel, broad-spectrum, pore-forming bacteriocin from Lactobacillus pentosus CS2 with quorum sensing regulatory mechanism and biopreservative potential. Front. Microbiol. 2018, 9, 564. [Google Scholar] [CrossRef]
- Jiang, H.; Zou, J.; Cheng, H.; Fang, J.; Huang, G. Purification, characterization, and mode of action of pentocin JL-1, a novel bacteriocin isolated from Lactobacillus pentosus, against drug-resistant Staphylococcus aureus. BioMed Res. Int. 2017, 2017, 7657190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darbandi, A.; Asadi, A.; Ari, M.M.; Ohadi, E.; Talebi, M.; Zadeh, M.H.; Emamie, A.D.; Ghanavati, R.; Kakanj, M. Bacteriocins: Properties and potential use as antimicrobials. J. Clin. Lab. Anal. 2021, 36, e24093. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Togawa, Y.; Shimosaka, M.; Okazaki, M. Purification and characterization of a novel bacteriocin produced by Lactobacillus crustorum MN047 isolated from koumiss from Xinjiang, China. J. Dairy Sci. 2016, 99, 7002–7015. [Google Scholar]
- Watthanasakphuban, N.; Tani, A.; Benjakul, S.; Maneerat, S. Detection and preliminary characterization of a narrow spectrum bacteriocin produced by Lactobacillus pentosus K2N7 from Thai traditional fermented shrimp (Kung-Som). J. Sci. Technol. 2016, 38, 47–55. [Google Scholar]
- Lv, X.; Ma, H.; Sun, M.; Lin, Y.; Bai, F.; Li, J.; Zhang, B. A novel bacteriocin DY4-2 produced by Lactobacillus plantarum from cutlassfish and its application as bio-preservative for the control of Pseudomonas fluorescens in fresh turbot (Scophthalmus maximus) fillets. Food Control 2018, 89, 22–31. [Google Scholar] [CrossRef]
- Yi, L.; Qi, T.; Ma, J.; Zeng, K. Genome and metabolites analysis reveal insights into control of foodborne pathogens in fresh-cut fruits by Lactobacillus pentosus MS031 isolated from Chinese Sichuan Paocai. Postharvest Biol. Technol. 2020, 164, 111150. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, S. Antibacterial Activity and Mechanism of Lacidophilin from Lactobacillus pentosus Against Staphylococcus aureus and Escherichia coli. Front. Microbiol. 2020, 11, 582349. [Google Scholar] [CrossRef] [PubMed]
- Cotter, P.D.; Ross, R.P.; Hill, C. Bacteriocins—A viable alternative to antibiotics? Nat. Rev. Microbiol. 2013, 11, 95–105. [Google Scholar] [CrossRef]
- Kumariya, R.; Garsa, A.K.; Rajput, Y.S.; Sood, S.K.; Akhtar, N.; Patel, S. Bacteriocins: Classification, synthesis, mechanism of action and resistance development in food spoilage causing bacteria. Microb. Pathog. 2019, 128, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Aizhan, R.; Yan, H.; Li, X.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Characterization, modes of action, and application of a novel broad-spectrum bacteriocin BM1300 produced by Lactobacillus crustorum MN047. Braz. J. Microbiol. 2020, 51, 2033–2048. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Ding, H.-P.; Tian, J.; Zhang, L.-L. Pore-forming mechanism of TUBP1 protein act on Verticillium dahliae. Process Biochem. 2018, 73, 6–14. [Google Scholar] [CrossRef]
- Wu, X.Z.; Chang, W.Q.; Cheng, A.X.; Sun, L.M.; Lou, H.X. Plagiochin E, an antifungal active macrocyclic bis (bibenzyl), induced apoptosis in Candida albicans through a metacaspase-dependent apoptotic pathway. BBA-Gen Subj. 2010, 1800, 439–447. [Google Scholar] [CrossRef]
- Zeng, H.; Li, T.; Tian, J.; Zhang, L.-L. TUBP1 protein lead to mitochondria-mediated apoptotic cell death in Verticillium dahliae. Int. J. Biochem. Cell Biol. 2018, 103, 35–44. [Google Scholar] [CrossRef]






| Strain Number | Inhibitory Zone Diameter/mm | MIC/mg·mL−1 | |
|---|---|---|---|
| 17-1 | 22.29 ± 0.15 | - | |
| 17-2 | 22.38 ± 0.69 | - | |
| 13-2 | 19.72 ± 0.69 | - | |
| 3-1 | 20.78 ± 0.62 | - | |
| 1-1 | 20.69 ± 0.10 | - | |
| Clinical isolates | 16-1 | 18.35 ± 0.72 | - |
| 3-2 | 18.74 ± 0.42 | - | |
| 9-2 | 21.73 ± 0.27 | - | |
| 13-1 | 17.99 ± 0.45 | - | |
| 16-2 | 17.67 ± 0.28 | - | |
| Standard strain | ATCC 29213 | 20.40 ± 0.56 | 31.25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, G.; Zeng, H. Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis. Molecules 2022, 27, 7627. https://doi.org/10.3390/molecules27217627
Wang G, Zeng H. Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis. Molecules. 2022; 27(21):7627. https://doi.org/10.3390/molecules27217627
Chicago/Turabian StyleWang, Gengchen, and Hong Zeng. 2022. "Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis" Molecules 27, no. 21: 7627. https://doi.org/10.3390/molecules27217627
APA StyleWang, G., & Zeng, H. (2022). Antibacterial Effect of Cell-Free Supernatant from Lactobacillus pentosus L-36 against Staphylococcus aureus from Bovine Mastitis. Molecules, 27(21), 7627. https://doi.org/10.3390/molecules27217627