Variable Dimensionality of Europium(III) and Terbium(III) Coordination Compounds with a Flexible Hexacarboxylate Ligand
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of the Coordination Compounds
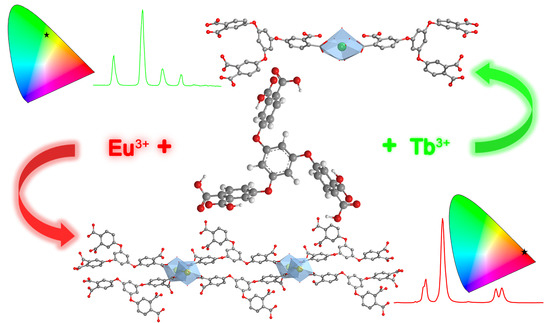
2.2. Crystal Structure of the Coordination Compounds
2.3. X-ray Powder Diffraction, IR Spectroscopy and Thermogravimetric Analysys
2.4. Luminescent Properties of the Ligand and the Coordinaion Compounds
3. Materials and Methods
3.1. Starting Materials and Synthetic Procedures
3.1.1. Synthesis of Compound [Tb(H4.5L)2(H2O)5]∙6H2O (1)
3.1.2. Synthesis of Compound {[Eu2(H3L)2(H2O)6]∙8H2O}n (2)
3.2. Physical Methods of Analysis
3.3. Single-Crystal X-ray Diffraction
3.4. Computational Chemistry Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Butova, V.V.; Soldatov, M.A.; Guda, A.A.; Lomachenko, K.A.; Lamberti, C. Metal-organic frameworks: Structure, properties, synthesis and characterization. Russ. Chem. Rev. 2016, 85, 280–307. [Google Scholar] [CrossRef]
- Belousov, Y.A.; Drozdov, A.A.; Taydakov, I.V.; Marchetti, F.; Pettinari, R.; Pettinari, C. Lanthanide azolecarboxylate compounds: Structure, luminescent properties and applications. Coord. Chem. Rev. 2021, 445, 214084. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Hanikel, N.; Yaghi, O.M. Secondary building units as the turning point in the development of the reticular chemistry of MOFs. Sci. Adv. 2022, 4, eaat9180. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.-Q.; Zhao, Y.; Zhang, X.-D.; Kang, Y.-S.; Lu, Q.-Y.; Azam, M.; Al-Resayes, S.I.; Sun, W.-Y. Metal–organic frameworks with 1,4-di(1H-imidazol-4-yl)benzene and varied carboxylate ligands for selectively sensing Fe(III) ions and ketone molecules. Dalton Trans. 2017, 46, 13943–13951. [Google Scholar] [CrossRef] [PubMed]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef] [PubMed]
- Guillerm, V.; Eddaoudi, M. The Importance of Highly Connected Building Units in Reticular Chemistry: Thoughtful Design of Metal–Organic Frameworks. Acc. Chem. Res. 2021, 54, 3298–3312. [Google Scholar] [CrossRef]
- Cheng, X.; Guo, L.; Wang, H.; Gu, J.; Yang, Y.; Kirillova, M.V.; Kirillov, A.M. Coordination Polymers from Biphenyl-Dicarboxylate Linkers: Synthesis, Structural Diversity, Interpenetration, and Catalytic Properties. Inorg. Chem. 2022, 61, 12577–12590. [Google Scholar] [CrossRef]
- Elsaidi, S.K.; Mohamed, M.H.; Banerjee, D.; Thallapally, P.K. Flexibility in Metal–Organic Frameworks: A fundamental understanding. Coord. Chem. Rev. 2018, 358, 125–152. [Google Scholar] [CrossRef]
- Murdock, C.R.; Lu, Z.; Jenkins, D.M. Effects of Solvation on the Framework of a Breathing Copper MOF Employing a Semirigid Linker. Inorg. Chem. 2013, 52, 2182–2187. [Google Scholar] [CrossRef]
- Bigdeli, F.; Lollar, C.T.; Morsali, A.; Zhou, H.-C. Switching in Metal–Organic Frameworks. Angew. Chemie Int. Ed. 2020, 59, 4652–4669. [Google Scholar] [CrossRef]
- Demakov, P.A.; Poryvaev, A.S.; Kovalenko, K.A.; Samsonenko, D.G.; Fedin, M.V.; Fedin, V.P.; Dybtsev, D.N. Structural Dynamics and Adsorption Properties of the Breathing Microporous Aliphatic Metal–Organic Framework. Inorg. Chem. 2020, 59, 15724–15732. [Google Scholar] [CrossRef] [PubMed]
- Albalad, J.; Peralta, R.A.; Huxley, M.T.; Tsoukatos, S.; Shi, Z.; Zhang, Y.-B.; Evans, J.D.; Sumby, C.J.; Doonan, C.J. Coordination modulated on-off switching of flexibility in a metal–organic framework. Chem. Sci. 2021, 12, 14893–14900. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Q.; Zhao, Y.; Wang, P.; Kang, Y.-S.; Azam, M.; Al-Resayes, S.I.; Liu, X.-H.; Lu, Q.-Y.; Sun, W.-Y. Fluorescent sensing and selective adsorption properties of metal–organic frameworks with mixed tricarboxylate and 1H-imidazol-4-yl-containing ligands. Dalton Trans. 2017, 46, 9022–9029. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, W.-J.; Gu, J.-Z.; Kirillova, M.V.; Kirillov, A.M. Zn(II) metal–organic architectures from ether-bridged tetracarboxylate linkers: Assembly, structural variety and catalytic features. CrystEngComm 2022, 24, 5297–5306. [Google Scholar] [CrossRef]
- Gu, J.; Wen, M.; Liang, X.; Shi, Z.; Kirillova, M.; Kirillov, A. Multifunctional Aromatic Carboxylic Acids as Versatile Building Blocks for Hydrothermal Design of Coordination Polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Gu, J.-Z.; Liang, X.-X.; Cui, Y.-H.; Wu, J.; Shi, Z.-F.; Kirillov, A.M. Introducing 2-(2-carboxyphenoxy)terephthalic acid as a new versatile building block for design of diverse coordination polymers: Synthesis, structural features, luminescence sensing, and magnetism. CrystEngComm 2017, 19, 2570–2588. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Gu, J.; Kirillova, M.V.; Kirillov, A.M. Introducing a flexible tetracarboxylic acid linker into functional coordination polymers: Synthesis, structural traits, and photocatalytic dye degradation. New J. Chem. 2020, 44, 16082–16091. [Google Scholar] [CrossRef]
- Bhadra, B.N.; Ahmed, I.; Lee, H.J.; Jhung, S.H. Metal-organic frameworks bearing free carboxylic acids: Preparation, modification, and applications. Coord. Chem. Rev. 2022, 450, 214237. [Google Scholar] [CrossRef]
- Hao, J.; Jikei, M.; Kakimoto, M. Preparation of Hyperbranched Aromatic Polyimides via A2 + B3 Approach. Macromolecules 2002, 35, 5372–5381. [Google Scholar] [CrossRef]
- Sahoo, S.; Mondal, S.; Sarma, D. Luminescent Lanthanide Metal Organic Frameworks (LnMOFs): A Versatile Platform towards Organomolecule Sensing. Coord. Chem. Rev. 2022, 470, 214707. [Google Scholar] [CrossRef]
- Wu, S.; Min, H.; Shi, W.; Cheng, P. Multicenter Metal–Organic Framework-Based Ratiometric Fluorescent Sensors. Adv. Mater. 2020, 32, 1805871. [Google Scholar] [CrossRef]
- Lunev, A.M.; Belousov, Y.A. Luminescent sensor materials based on rare-earth element complexes for detecting cations, anions, and small molecules. Russ. Chem. Bull. 2022, 71, 825–857. [Google Scholar] [CrossRef]
- Song, D.; Ji, X.; Li, Y.; Chen, S.; Wu, S.; Zhang, Y.; Gao, E.; Zhu, M. A terbium-based coordination polymer for sensitive ratiometric fluorescence detection of lamotrigine. J. Lumin. 2022, 251, 119129. [Google Scholar] [CrossRef]
- Ma, Y.; Zhu, M.; Zhang, Y.; Sun, Y.; Wu, S. A water-stable Eu-MOF as multi-responsive luminescent sensor for high-efficiency detection of Fe3+, MnO4− ions and nicosulfuron in aqueous solution. J. Solid State Chem. 2022, 316, 123598. [Google Scholar] [CrossRef]
- Demakov, P.A.; Ryadun, A.A.; Dorovatovskii, P.V.; Lazarenko, V.A.; Samsonenko, D.G.; Brylev, K.A.; Fedin, V.P.; Dybtsev, D.N. Intense multi-colored luminescence in a series of rare-earth metal-organic frameworks with aliphatic linkers. Dalton Trans. 2021, 50, 11899–11908. [Google Scholar] [CrossRef] [PubMed]
- Smirnova, K.S.; Ivanova, E.A.; Pozdnyakov, I.P.; Russkikh, A.A.; Eltsov, I.V.; Dotsenko, V.V.; Lider, E.V. 2D polymeric lanthanide(III) compounds based on novel bright green emitting enaminoneligand. Inorg. Chim. Acta 2022, 542, 121107. [Google Scholar] [CrossRef]
- Khistiaeva, V.V.; Melnikov, A.S.; Slavova, S.O.; Sizov, V.V.; Starova, G.L.; Koshevoy, I.O.; Grachova, E.V. Heteroleptic β-diketonate Ln(iii) complexes decorated with pyridyl substituted pyridazine ligands: Synthesis, structure and luminescence properties. Inorg. Chem. Front. 2018, 5, 3015–3027. [Google Scholar] [CrossRef]
- Shi, X.; Cao, B.; Liu, J.; Zhang, J.; Du, Y. Rare-Earth-Based Metal–Organic Frameworks as Multifunctional Platforms for Catalytic Conversion. Small 2021, 17, 2005371. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Zhao, Z.-S.; Wang, Z.; Zhang, R.; Liu, L.; Han, Z.-B. Recent progress in lanthanide metal–organic frameworks and their derivatives in catalytic applications. Inorg. Chem. Front. 2021, 8, 590–619. [Google Scholar] [CrossRef]
- Biswas, S.; Neugebauer, P. Lanthanide-Based Metal-Organic-Frameworks for Proton Conduction and Magnetic Properties. Eur. J. Inorg. Chem. 2021, 2021, 4610–4618. [Google Scholar] [CrossRef]
- Feng, L.; Wang, H.-S.; Xu, H.-L.; Huang, W.-T.; Zeng, T.-Y.; Cheng, Q.-R.; Pan, Z.-Q.; Zhou, H. A water stable layered Tb(III) polycarboxylate with high proton conductivity over 10−2 S cm−1 in a wide temperature range. Chem. Commun. 2019, 55, 1762–1765. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, S.; Qu, X.; Yang, Q.; Liu, X.; Wei, Q.; Xie, G.; Chen, S. Synthesis, crystal structure and photoluminescence property of Eu/Tb MOFs with mixed polycarboxylate ligands. J. Solid State Chem. 2015, 231, 223–229. [Google Scholar] [CrossRef]
- Alemany, P.; Casanova, D.; Alvarez, S.; Dryzun, C.; Avnir, D. Continuous Symmetry Measures: A New Tool in Quantum Chemistry. Rev. Comput. Chem. 2017, 30, 289–352. [Google Scholar]
- Ruiz-Martínez, A.; Casanova, D.; Alvarez, S. Polyhedral Structures with an Odd Number of Vertices: Nine-Coordinate Metal Compounds. Chem. A Eur. J. 2008, 14, 1291–1303. [Google Scholar] [CrossRef] [PubMed]
- Demakov, P.A.; Ryadun, A.A.; Fedin, V.P. Aliphatic-Bridged Early Lanthanide Metal–Organic Frameworks: Topological Polymorphism and Excitation-Dependent Luminescence. Inorganics 2022, 10, 163. [Google Scholar]
- Demakov, P.A.; Sapchenko, S.A.; Samsonenko, D.G.; Dybtsev, D.N.; Fedin, V.P. Gadolinium Break in a Series of Three-Dimensional trans-1,4-Cyclohexane Dicarboxylates of Rare Earth Elements. J. Struct. Chem. 2019, 60, 815–822. [Google Scholar] [CrossRef]
- Tan, X.-F.; Zhou, J.; Zou, H.-H.; Fu, L.; Tang, Q.; Wang, P. A series of lanthanide glutarates: Lanthanide contraction effect on crystal frameworks of lanthanide glutarates. RSC Adv. 2017, 7, 17934–17940. [Google Scholar] [CrossRef] [Green Version]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [Green Version]
- Binnemans, K. Interpretation of europium(III) spectra. Coord. Chem. Rev. 2015, 295, 1–45. [Google Scholar] [CrossRef] [Green Version]
- Alzakhem, N.; Bischof, C.; Seitz, M. Dependence of the Photophysical Properties on the Number of 2,2′-Bipyridine Units in a Series of Luminescent Europium and Terbium Cryptates. Inorg. Chem. 2012, 51, 9343–9349. [Google Scholar] [CrossRef]
- Huang, Y.; Zhu, Q.; Sheng, T.; Hu, S.; Fu, R.; Shen, C.; Tan, C.; Wen, Y.; Bai, S.; Wu, X. Lanthanide coordination polymers assembled from triazine-based flexible polycarboxylate ligands and their luminescent properties. CrystEngComm 2013, 15, 3560–3567. [Google Scholar] [CrossRef]
- Gai, Y.; Jiang, F.; Chen, L.; Wu, M.; Su, K.; Pan, J.; Wan, X.; Hong, M. Europium and Terbium Coordination Polymers Assembled from Hexacarboxylate Ligands: Structures and Luminescent Properties. Cryst. Growth Des. 2014, 14, 1010–1017. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. Sect. A 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Rev. D.01; Gaussian, Inc.: Wallingford, CT, USA, 2013. [Google Scholar]
- Becke, A.D. Density-functional exchange-energy approximation with correct asymptotic behavior. Phys. Rev. A 1988, 38, 3098–3100. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef] [Green Version]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Frisch, M.J.; Pople, J.A.; Binkley, J.S. Self-consistent molecular orbital methods 25. Supplementary functions for Gaussian basis sets. J. Chem. Phys. 1984, 80, 3265–3269. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef]







| Parameter | Compound 1 | Compound 2 |
|---|---|---|
| Molecular formula | C60H43O35Tb | C30H29EuO22 |
| Formula weight, g∙mol−1 | 1481.85 | 893.49 |
| Temperature, K | 150(2) | 150.00 |
| Crystal system | Monoclinic | Triclinic |
| Space group | C2/c | P-1 |
| a, Å | 38.585(2) | 8.66180(10) |
| b, Å | 13.1992(7) | 10.0305(2) |
| c, Å | 11.9356(7) | 19.2763(3) |
| α/° | 90 | 87.9920(10) |
| β, ° | 106.289(2) | 80.1830(10) |
| γ/° | 90 | 87.1930(10) |
| Cell volume, Å | 5834.6(6) | 1647.66(5) |
| Z | 4 | 2 |
| ρcalc, g/cm3 | 1.687 | 1.801 |
| μ, mm−1 | 1.320 | 2.000 |
| F(000) | 2988.0 | 896.0 |
| Independent reflections | 9710 [Rint = 0.0508, Rsigma = 0.0445] | 5809 [Rint = 0.0405, Rsigma = 0.0402] |
| Goodness-of-fit on F2 | 1.061 | 1.036 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0353, wR2 = 0.0756 | R1 = 0.0263, wR2 = 0.0547 |
| Final R indexes [all data] | R1 = 0.0458, wR2 = 0.0786 | R1 = 0.0295, wR2 = 0.0565 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, X.; Pavlov, D.I.; Ryadun, A.A.; Potapov, A.S.; Fedin, V.P. Variable Dimensionality of Europium(III) and Terbium(III) Coordination Compounds with a Flexible Hexacarboxylate Ligand. Molecules 2022, 27, 7849. https://doi.org/10.3390/molecules27227849
Yu X, Pavlov DI, Ryadun AA, Potapov AS, Fedin VP. Variable Dimensionality of Europium(III) and Terbium(III) Coordination Compounds with a Flexible Hexacarboxylate Ligand. Molecules. 2022; 27(22):7849. https://doi.org/10.3390/molecules27227849
Chicago/Turabian StyleYu, Xiaolin, Dmitry I. Pavlov, Alexey A. Ryadun, Andrei S. Potapov, and Vladimir P. Fedin. 2022. "Variable Dimensionality of Europium(III) and Terbium(III) Coordination Compounds with a Flexible Hexacarboxylate Ligand" Molecules 27, no. 22: 7849. https://doi.org/10.3390/molecules27227849
APA StyleYu, X., Pavlov, D. I., Ryadun, A. A., Potapov, A. S., & Fedin, V. P. (2022). Variable Dimensionality of Europium(III) and Terbium(III) Coordination Compounds with a Flexible Hexacarboxylate Ligand. Molecules, 27(22), 7849. https://doi.org/10.3390/molecules27227849